Investigation of Vitamin D Receptor Gene Polymorphism in Pediatric Patients with Brain Cancer
CC BY-NC-ND 4.0 · Indian J Med Paediatr Oncol 2017; 38(02): 128-132
DOI: DOI: 10.4103/ijmpo.ijmpo_22_16
Abstract
Aim: In recent years, it is believed that Vitamin D may play a protective role in some cancer types. Certain regions of the Vitamin D receptor (VDR) gene may show a genetic difference in structure. The most frequent polymorphisms in this gene are in Taq-1, Fok-1, and Bsm-1 regions. Some adult cancer types are associated with VDR gene polymorphism such as; colorectal carcinoma, breast carcinoma, and prostate carcinoma. Reviewing the medical literature, no such study had been done on children so far. Materials and Methods: We investigated the association of the three most common gene polymorphisms (Taq-1, Fok-1, and Bsm-1 regions) in VDR gene in 32 children with brain tumors and forty control healthy volunteers. Results: We could not find any relationship between childhood brain tumors and VDR gene polymorphism in these three regions. Conclusion: The present results suggest that the Taq-1, Fok-1, and Bsm-1 polymorphism in the VDR gene and pediatric brain cancers have no association.
Publication History
Article published online:
06 July 2021
© 2017. Indian Society of Medical and Paediatric Oncology. This is an open access article published by Thieme under the terms of the Creative Commons Attribution-NonDerivative-NonCommercial-License, permitting copying and reproduction so long as the original work is given appropriate credit. Contents may not be used for commercial purposes, or adapted, remixed, transformed or built upon. (https://creativecommons.org/licenses/by-nc-nd/4.0/.)
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India
Abstract
Aim:
In recent years, it is believed that Vitamin D may play a protective role in some cancer types. Certain regions of the Vitamin D receptor (VDR) gene may show a genetic difference in structure. The most frequent polymorphisms in this gene are in Taq-1, Fok-1, and Bsm-1 regions. Some adult cancer types are associated with VDR gene polymorphism such as; colorectal carcinoma, breast carcinoma, and prostate carcinoma. Reviewing the medical literature, no such study had been done on children so far.
Materials and Methods:
We investigated the association of the three most common gene polymorphisms (Taq-1, Fok-1, and Bsm-1 regions) in VDR gene in 32 children with brain tumors and forty control healthy volunteers.
Results:
We could not find any relationship between childhood brain tumors and VDR gene polymorphism in these three regions.
Conclusion:
The present results suggest that the Taq-1, Fok-1, and Bsm-1 polymorphism in the VDR gene and pediatric brain cancers have no association.
Introduction
Vitamin D is a steroid in structure. It bounds to special receptors and plays an important role in cell proliferation and inflammation.[1] Certain regions of the Vitamin D receptor (VDR) gene may show a genetic difference in structure. The most frequent polymorphisms in this gene are in Bsm-1 [Figure 1], Fok-1 [Figure 2], and Taq-1 [Figure 3] regions.
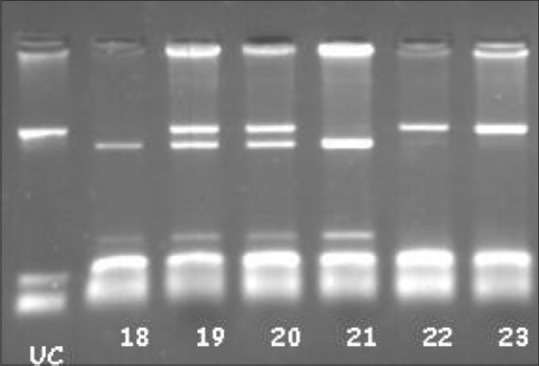
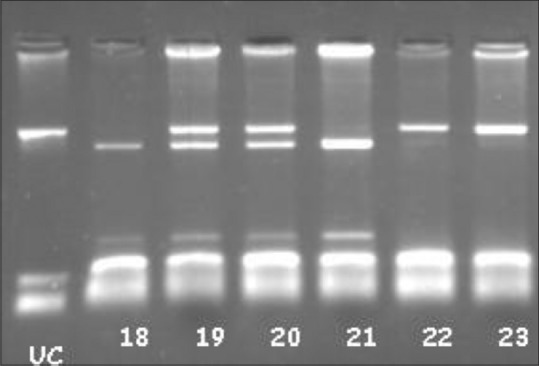
| Figure 1Vitamin D receptor BsmI polymorphism samples: UC: Un Cut. 18 and 21 bb, 19 and 20 Bb, 22–23 BB
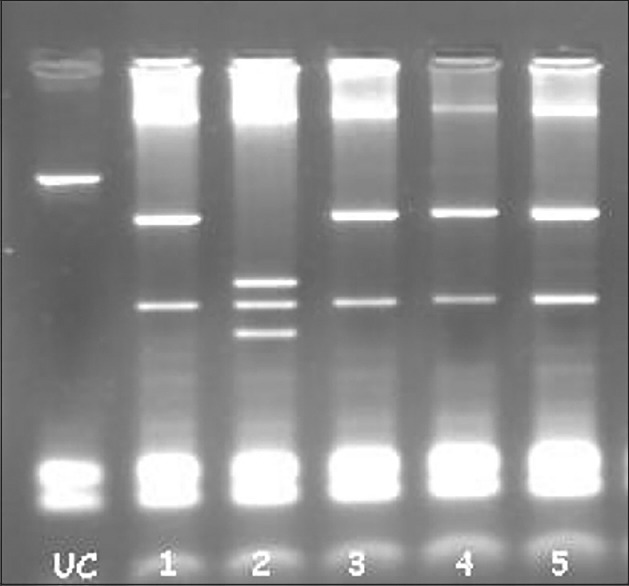
| Figure 2Vitamin D receptor FokI polymorphism rif-lip genotyping. UC: Un Cut FF: 30, Ff; 26, 27, 28, 29 and 31 (Samples)
| Figure 3:VDR TaqI polymorphism. UC: Un cut. CC: 2. Sample TT: except 2 (all samples)
In recent years, the relevance of VDR gene restriction fragment length polymorphisms for various types of cancer has been investigated by a great number of studies.[2] It has been hypothesized that VDR polymorphisms may influence both the risk of cancer occurrence and prognosis. After careful evaluation of the actual literature, it can be summarized that data indicating an association of VDR polymorphisms and cancer risk are strongest for breast cancer (Bsm1, Fok1), prostate cancer (Fok1), and malignant melanoma (MM) (Fok1). Data indicating an association of VDR polymorphisms and cancer prognosis are strongest for prostate cancer (Fok1), breast cancer (Bsm1, Taq1), MM (Bsm1), and renal cell carcinoma (Taq1).[2,3,4,5,6,7,8,9,10,11,12,13]
When we reviewed the medical literature, we could not find any studies, investigating the relationship between VDR polymorphism and pediatric cancers. For this reason, we planned to investigate the association of VDR polymorphism and childhood brain malignant tumors.
Materials and Methods
This study was performed on 32 volunteer patients between ages 0 and 18 who were diagnosed with brain tumor by radiological or pathological modalities. All of them were being followed up by Marmara University Paediatric Hematology and Oncology Department. As a control group, forty volunteer patients between ages 0 and 18 who had admitted to Marmara University Pediatric Policlinics for other reasons than a chronic illness were chosen.
All patients and/or guardians participating in this study confirmed informed consent following institutional guidelines in accordance with the Declaration of Helsinki. The study was approved by Marmara University Ethics Committee.
DNA isolations
Five milliliters whole blood samples were taken into 0.5 M ethylenediaminetetraacetic acid (EDTA) tube (Sigma, USA) from the individuals. All samples were mixed up with red blood cell (RBC) lysis solutions (155 mM ammonium chloride [AppliChem, Germany]), 10 mM sodium bicarbonate (Merck, Germany), and 0.5 mM EDTA (AppliChem, Germany) and incubated at 20° for 20 min.
Moreover, this solution was centrifuged for 20 min at +4°C, 4000 rpm (Hettich, Germany). Then, supernatant was removed, and residual liquid was mixed with RBC lysis solution. This procedure was repeated until all erythrocytes lysed and removed. Residual leukocytes were mixed with 1000 mcl RBC lysis solution; 800 mcl of it was kept in the Eppendorf tube. 20 μ/ml proteinase K (MBI Fermentas, Lithuania), sodium dodecyl sulfate 10% (Merck, Germany) in final concentration %0.5, nuclease solution (10 mM Trichloride [Amresco, USA]) pH: 8, 100 mM sodium chloride (Merck, Germany) in an amount that two and a half times the volume of leukocytes, 1 mM EDTA (AppliChem, Germany) added into the residual 200 mcl solution, and incubated over a night in +56°C hot water (Kottermann Labortechnik, Germany). On the 2nd day, 1:1 phenol/chloroform (Merck, Germany) and isoamyl alcohol (isopentyl alcohol) were added and shaked for 10 min. The sample was held in the ice for 20 min and centrifuged at +4°C, 4000 rpm for 20 min. The solution was splitted up into two parts. The upper portion was taken into an another Eppendorf tube. 3 M sodium acetate (Sigma, USA) as much as 1/10 volume of solution and in an amount that is two times the volume of solution 95% alcohol (Tekel, Turkey) was added. After the tube upturned, DNA became visible and incubated at 20°C over the night. Centrifuged DNA at +4°C and 4000 rpm for 20 min precipitated. Supernatant part throwed away. 500 mcl 70% alcohol was added and centrifuged +4°C 4000 rpm for 20 min. At the end of centrifugation, alcohol was poured and let the tube dry. After drying, tris-EDTA (10 mM Tris-Hcl, 1 mM EDTA) was added and incubated over the night at 37°C to let the DNA dissolve.
Screening for Vitamin D receptor gene FokI (rs10735810), BsmI (rs1544410), and TaqI (rs731236) polymorphism
For the analysis of VDR gene, polymorphism polymerase chain reaction (PCR) - restriction fragment length polymorphism method was used. Other PCR components are 10 mM tris-HCl (25°C pH: 8.8), 50 mM KCl, arranged last concentrations 0.2 mM deoxynucleoside triphosphates deoxyadenosine triphosphate (dATP), deoxyguanosine triphosphate (dGTP), deoxycytidine triphosphate (dCTP), deoxythymidine triphosphate (dTTP) (Fermentas, Lithuania), and 15 mM MgCl2. PCR is implemented by completing total volume to 25 mcl with deionized H2O.
VDR gene BsmI polymorphism region;
5’-CAA CCA AGA CTA CAA GTA CCG CGT CAG TGA-3’
5’-AAC CAG CGG GAA GAG GTC AAG GG-3’
FokI polymorphism region;
5’-AGC TGG CCC TGG CAC TGA CTC TGC TCT-3’
5’-ATG GAA ACA CCT TGC TTC TTC TCC CTC-3’
TaqI polymorphism region;
5’-CAG AGC ATGGAC AGG GAG CAA-3’
5’-GCA ACT CCT CAT GGC TGA GGT C CTC-3’
By using those primers, gene region multiplied through PCR. After amplification with PCR method, BsmI polymorphism was found 825 base pairs (bp), FokI polymorphism was found 265 bp, TaqI polymorphism was found 740 bp.
Temperature conditions for PCR are 5 min denaturation at 95°C, 1 min forty cycles denaturation at 95°C, 1 min hybridization at 60°C, elongation at 72°C, and 7 min last elongation was implemented (T100, Bio-Rad).
After PCR experiment, products of PCR are loaded to 2% agarose gel electrophoresis and controlled; if the correct gene region amplification is seen, cutting process was performed by using restriction endonuclease enzyme.
Agarose gel electrophoresis
Cutting results of endonuclease restriction enzyme are evaluated in 3% agarose gel electrophoresis. Products, which were cut with matching enzyme, treated with orange G, load to the gel and patterns moved at 90–100 V (Biogen, USA) for 30–50 min and results evaluated under ultraviolet light.
The statistical package, SPSS 17.0 (SPSS Inc, Chicago, USA), was used to analyze the data. The allele groups were compared to chi-square test and P < 0>
A total of 32 patients between ages 0 and 18 with a diagnosis of brain tumor chosen for the study group. Control group consisted of forty healthy volunteers without any chronic illness [Table 1].
Table 1
Gender distribution of the study and control groups

|
There was no statistically significant difference in gender distribution between two groups (P = 0.96).
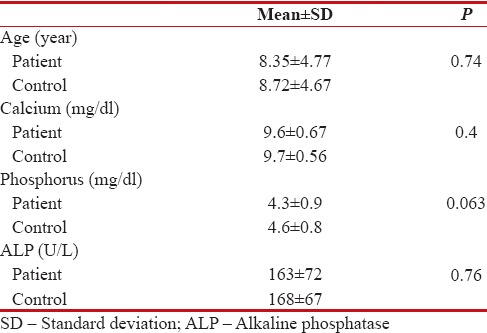
The median patient age was 8.35 ± 4.77 years old; for the control group, it was 8.72 ± 4.67 years old. There was no statistically significant difference in age distribution between two groups (P = 0.74).
Calcium (Ca), phosphorus (P), and alkaline phosphatase (ALP) levels are indicators of Ca metabolism in serum. Ca level was 9.6 ± 0.67 mg/dl in patient group and 9.7 ± 0.56 mg/dl in control group (P = 0.4). P level was 4.3 ± 0.9 mg/dl in patient group and 4.6 ± 0.8 mg/dl in control group (P = 0.063). ALP level was 163 ± 72 U/L in patient group and 168 ± 67 U/L in control group (P = 0.76). There was no statistically significant difference in Ca, P, ALP levels between two groups [Tables [Tables22 and and33].
Table 2
Age, calcium, phosphorus, alkaline phosphatase levels of patient and control groups
|
Table 3
Histological dispersion of brain tumors

|
Evaluation of Vitamin D receptor gene polymorphisms
Vitamin D receptor, Bsm1 polymorphism
When evaluation was made in terms of Bsm1 gene polymorphism, bb allele was 8/32 in patient group and 11/40 in control group. The distribution of bb allele was similar between two groups (P = 0.97) [Table 4].
Table 4
Bsm1 gene polymorphism, distribution of patient and control groups

|
Vitamin D receptor, Fok1 polymorphism
No one was carrying ff allele among patients. One person was found with ff allele in control group [Table 5].
Table 5
Fok1 gene polymorphism, distribution of the study and control groups

|
There was no significant difference between the patient and control groups in terms of Fok1 polymorphism.
Vitamin D receptor, Tak1 polymorphism
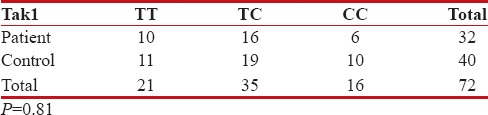
For Tak1 polymorphism, CC was positive in 6/32 of the patients and 10/40 of the control group. There was no significant difference statistically between the patient and control groups in terms of VDR Tak1 polymorphism (P = 0.81) [Table 6].
Table 6
Tak1 gene polymorphism, distribution of the study and control groups
|
As a result, we could not find any relationship between childhood brain tumors and VDR gene polymorphism in these three regions.
Discussion
Vitamin D is an important factor in the regulation of cell division and differentiation. The VDR gene is a member of nuclear receptor superfamily, and it is located on chromosome 12.
For a long time, several polymorphisms in VDR gene have been investigated for functional significance and potential effects on disease susceptibility.[14] Many studies reported that Vitamin D has an antiproliferative effect on many cancer types, which is promoting apoptosis in a variety of malignant cells, such as glioma, neuroblastoma, leukemia, lymphoma cells, breast cancer, and colon cancer,[3,15,16] and numerous Vitamin D analogs have been produced for the treatment of several cancer types. Alternations of Vitamin D levels may be related to the changing in the expression of several transcription factors, cell cycle arrested proteins, growth factor, and other genes.[17] Several studies implicated that the ff and Ff genotypes of the VDR gene are associated with a decreased transcriptional activity. VDR Fok-I polymorphism changes the size of the VDR protein.[18,19,20,21] The shorter VDR variant could be less active; therefore, this variation may lead to more aggressive disease prognosis.[15] There are some studies showing that Vitamin D deficiency affected the brain morphology and cellular proliferation and growth factor signaling in other tissue.[22,23,24,25]
VDR expression rates were associated with KRAS mutation in several cancer types. Several studies suggest that cellular effects of VDR may be associated with MAPK signaling pathways, especially KRAS mutation in several cancer types such as breast and colorectal cancers.[26,27] Sutton et al. demonstrated that protein levels of MN1 were significantly in a relationship with Vitamin D-mediated transcription mechanism in osteoblastic cells.[17]
It has been reported that VDRs localized in neuronal and glial cells affect the metabolism of brain cells and change the expression of VDR. Synthetic Vitamin D analogs are among the preferred options in the treatment of CNS tumors. In addition, phase II clinical studies have correlated with positive effects of Vitamin D therapy on glioblastoma cells.[23,29,30,31,32,33,34]
In their study, Toptas et al. found that there was statistically significant difference between the control and meningioma patients for Fok-I ff genotypes. The individuals who had VDR Fok-I ff genotype had an increased risk for meningioma.[35]
We think that there is not enough study about relationship between childhood cancers and VDR gene polymorphism, when the medical literature is reviewed. In our study, we investigated the association of the three most common gene polymorphisms (Taq-1, Fok-1, and Bsm-1 regions) in VDR gene in 32 children with brain malignant tumors.
We could not find any relationship between childhood brain tumors and VDR gene polymorphism in these three regions.
Conclusion
The present results suggested that the Taq-1, Fok-1, and Bsm-1 polymorphism in the VDR gene and pediatric brain cancers have no association. However, further studies with large number of individuals are needed in that subject.
Financial support and sponsorship
This study was supported by Marmara University Scientific Research Fund, and this study was approved by Marmara University Ethics Committee.
Conflicts of interest
There are no conflicts of interest.
References
- Haussler MR, Whitfield GK, Kaneko I, Haussler CA, Hsieh D, Hsieh JC, et al. Molecular mechanisms of Vitamin D action. Calcif Tissue Int 2013;92:77-98.
- Clevers H. At the crossroads of inflammation and cancer. Cell 2004;118:671-4.
- Giovannucci E. The epidemiology of Vitamin D and cancer incidence and mortality: A review (United States). Cancer Causes Control 2005;16:83-95.
- Freedman DM, Looker AC, Chang SC, Graubard BI. Prospective study of serum Vitamin D and cancer mortality in the United States. J Natl Cancer Inst 2007;99:1594-602.
- Moore CE, Murphy MM, Holick MF. Vitamin D intakes by children and adults in the United States differ among ethnic groups. J Nutr 2005;135:2478-85.
- Garland CF, Gorham ED, Mohr SB, Garland FC. Vitamin D for cancer prevention: Global perspective. Ann Epidemiol 2009;19:468-83.
- Colston K, Colston MJ, Feldman D. 1,25-dihydroxyvitamin D3 and malignant melanoma: The presence of receptors and inhibition of cell growth in culture. Endocrinology 1981;108:1083-6.
- Miyaura C, Abe E, Kuribayashi T, Tanaka H, Konno K, Nishii Y, et al. 1 alpha, 25-dihydroxyvitamin D3 induces differentiation of human myeloid leukemia cells. Biochem Biophys Res Commun 1981;102:937-43.
- Honma Y, Hozumi M, Abe E, Konno K, Fukushima M, Hata S, et al. 1 alpha, 25-dihydroxyvitamin D3 and 1 alpha-hydroxyvitamin D3 prolong survival time of mice inoculated with myeloid leukemia cells. Proc Natl Acad Sci U S A 1983;80:201-4.
- Mazilli SA, Reid ME, Foster BA. Vitamin D and cancer chemoprevention. In: Trump DL, Johnson CS, editors. Vitamin D and Cancer. 1st ed. New York: Springer Science + Business Mediae; 2011. p. 175-89.
- ;Gorham ED, Garland CF, Garland FC, Grant WB, Mohr SB, Lipkin M, et al. Optimal Vitamin D status for colorectal cancer prevention: A quantitative meta analysis. Am J Prev Med 2007;32:210-6.
- Gorham ED, Garland CF, Garland FC, Grant WB, Mohr SB, Lipkin M,et al. Vitamin D and prevention of colorectal cancer. J Steroid Biochem Mol Biol 2005;97:179-94.
- Bjelakovic G, Gluud LL, Nikolova D, Whitfield K, Wetterslev J, Gluud C. Vitamin D supplementation for prevention of cancer in adults. Cochrane Database of Systematic Reviews 2008:CD007469.
- Mittal RD, Manchanda PK, Bhat S, Bid HK. Association of Vitamin-D receptor (Fok-I) gene polymorphism with bladder cancer in an Indian population. BJU Int 2007;99:933-7.
- Raimondi S, Johansson H, Maisonneuve P, Gandini S. Review and meta-analysis on Vitamin D receptor polymorphisms and cancer risk. Carcinogenesis 2009;30:1170-80.
- Eyles D, Brown J, Mackay-Sim A, McGrath J, Feron F. Vitamin D3 and brain development. Neuroscience 2003;118:641-53.
- Sutton AL, Zhang X, Ellison TI, Macdonald PN. The 1,25(OH) 2D3-regulated transcription factor MN1 stimulates Vitamin D receptor-mediated transcription and inhibits osteoblastic cell proliferation. Mol Endocrinol 2005;19:2234-44.
- Arai H, Miyamoto K, Taketani Y, Yamamoto H, Iemori Y, Morita K, et al. AVitamin D receptor gene polymorphism in the translation initiation codon: Effect on protein activity and relation to bone mineral density in Japanese women. J Bone Miner Res 1997;12:915-21.
- Swapna N, Vamsi UM, Usha G, Padma T. Risk conferred by FokI polymorphism of Vitamin D receptor (VDR) gene for essential hypertension. Indian J Hum Genet 2011;17:201-6.
- Jurutka PW, Remus LS, Whitfield GK, Thompson PD, Hsieh JC, Zitzer H, et al. The polymorphic N terminus in human Vitamin D receptor isoforms influences transcriptional activity by modulating interaction with transcription factor IIB. Mol Endocrinol 2000;14:401-20.
- Colin EM, Weel AE, Uitterlinden AG, Buurman CJ, Birkenhäger JC, Pols HA, et al. Consequences of Vitamin D receptor gene polymorphisms for growth inhibition of cultured human peripheral blood mononuclear cells by 1, 25-dihydroxyvitamin D3. Clin Endocrinol (Oxf) 2000;52:211-6.
- Sweeney C, Curtin K, Murtaugh MA, Caan BJ, Potter JD, Slattery ML. Haplotype analysis of common Vitamin D receptor variants and colon and rectal cancers. Cancer Epidemiol Biomarkers Prev 2006;15:744-9.
- Garcion E, Wion-Barbot N, Montero-Menei CN, Berger F, Wion D. New clues about Vitamin D functions in the nervous system. Trends Endocrinol Metab 2002;13:100-5.
- ;McGrath JJ, Féron FP, Burne TH, Mackay-Sim A, Eyles DW. Vitamin D3-implications for brain development. J Steroid Biochem Mol Biol 2004;89-90:557-60.
- Tseng M, Breslow RA, Graubard BI, Ziegler RG. Dairy, calcium, and Vitamin D intakes and prostate cancer risk in the National Health and Nutrition Examination Epidemiologic Follow-up Study cohort. Am J Clin Nutr 2005;81:1147-54.
- Feskanich D, Ma J, Fuchs CS, Kirkner GJ, Hankinson SE, Hollis BW, et al. Plasma Vitamin D metabolites and risk of colorectal cancer in women. Cancer Epidemiol Biomarkers Prev 2004;13:1502-8.
- Qi X, Pramanik R, Wang J, Schultz RM, Maitra RK, Han J, et al. The p38 and JNK pathways cooperate to trans-activate Vitamin D receptor via c-Jun/AP-1 and sensitize human breast cancer cells to Vitamin D(3)-induced growth inhibition. J Biol Chem 2002;277:25884-92.
- Kure S, Nosho K, Baba Y, Irahara N, Shima K, Ng K, et al. Vitamin D receptor expression is associated with PIK3CA and KRAS mutations in colorectal cancer. Cancer Epidemiol Biomarkers Prev 2009;18:2765-72.
- Naveilhan P, Berger F, Haddad K, Barbot N, Benabid AL, Brachet P, et al. Induction of glioma cell death by 1,25(OH) 2 Vitamin D3: Towards an endocrine therapy of brain tumors? J Neurosci Res 1994;37:271-7.
- Baudet C, Chevalier G, Naveilhan P, Binderup L, Brachet P, Wion D. Cytotoxic effects of 1 alpha, 25-dihydroxyvitamin D3 and synthetic Vitamin D3 analogues on a glioma cell line. Cancer Lett 1996;100:3-10.
- Baudet C, Chevalier G, Chassevent A, Canova C, Filmon R, Larra F, et al. 1,25-Dihydroxyvitamin D3 induces programmed cell death in a rat glioma cell line. J Neurosci Res 1996;46:540-50.
- Davoust N, Wion D, Chevalier G, Garabedian M, Brachet P, Couez D. Vitamin D receptor stable transfection restores the susceptibility to 1,25-dihydroxyvitamin D3 cytotoxicity in a rat glioma resistant clone. J Neurosci Res 1998;52:210-9.
- Trouillas P, Honnorat J, Bret P, Jouvet A, Gerard JP. Redifferentiation therapy in brain tumors: Long-lasting complete regression of glioblastomas and an anaplastic astrocytoma under long term 1-alpha-hydroxycholecalciferol. J Neurooncol 2001;51:57-66.
- Neveu I, Naveilhan P, Menaa C, Wion D, Brachet P, Garabédian M. Synthesis of 1,25-dihydroxyvitamin D3 by rat brain macrophages in vitro. J Neurosci Res 1994;38:214-20.
- Toptas B, Kafadar AM, Cacina C, Turan S, Yurdum LM, Yigitbasi N, et al. The Vitamin D receptor (VDR) gene polymorphisms in Turkish brain cancer patients. Biomed Res Int 2013;2013:295791.

| Figure 1Vitamin D receptor BsmI polymorphism samples: UC: Un Cut. 18 and 21 bb, 19 and 20 Bb, 22–23 BB

| Figure 2Vitamin D receptor FokI polymorphism rif-lip genotyping. UC: Un Cut FF: 30, Ff; 26, 27, 28, 29 and 31 (Samples)

| Figure 3:VDR TaqI polymorphism. UC: Un cut. CC: 2. Sample TT: except 2 (all samples)
References
- Haussler MR, Whitfield GK, Kaneko I, Haussler CA, Hsieh D, Hsieh JC, et al. Molecular mechanisms of Vitamin D action. Calcif Tissue Int 2013;92:77-98.
- Clevers H. At the crossroads of inflammation and cancer. Cell 2004;118:671-4.
- Giovannucci E. The epidemiology of Vitamin D and cancer incidence and mortality: A review (United States). Cancer Causes Control 2005;16:83-95.
- Freedman DM, Looker AC, Chang SC, Graubard BI. Prospective study of serum Vitamin D and cancer mortality in the United States. J Natl Cancer Inst 2007;99:1594-602.
- Moore CE, Murphy MM, Holick MF. Vitamin D intakes by children and adults in the United States differ among ethnic groups. J Nutr 2005;135:2478-85.
- Garland CF, Gorham ED, Mohr SB, Garland FC. Vitamin D for cancer prevention: Global perspective. Ann Epidemiol 2009;19:468-83.
- Colston K, Colston MJ, Feldman D. 1,25-dihydroxyvitamin D3 and malignant melanoma: The presence of receptors and inhibition of cell growth in culture. Endocrinology 1981;108:1083-6.
- Miyaura C, Abe E, Kuribayashi T, Tanaka H, Konno K, Nishii Y, et al. 1 alpha, 25-dihydroxyvitamin D3 induces differentiation of human myeloid leukemia cells. Biochem Biophys Res Commun 1981;102:937-43.
- Honma Y, Hozumi M, Abe E, Konno K, Fukushima M, Hata S, et al. 1 alpha, 25-dihydroxyvitamin D3 and 1 alpha-hydroxyvitamin D3 prolong survival time of mice inoculated with myeloid leukemia cells. Proc Natl Acad Sci U S A 1983;80:201-4.
- Mazilli SA, Reid ME, Foster BA. Vitamin D and cancer chemoprevention. In: Trump DL, Johnson CS, editors. Vitamin D and Cancer. 1st ed. New York: Springer Science + Business Mediae; 2011. p. 175-89.
- ;Gorham ED, Garland CF, Garland FC, Grant WB, Mohr SB, Lipkin M, et al. Optimal Vitamin D status for colorectal cancer prevention: A quantitative meta analysis. Am J Prev Med 2007;32:210-6.
- Gorham ED, Garland CF, Garland FC, Grant WB, Mohr SB, Lipkin M,et al. Vitamin D and prevention of colorectal cancer. J Steroid Biochem Mol Biol 2005;97:179-94.
- Bjelakovic G, Gluud LL, Nikolova D, Whitfield K, Wetterslev J, Gluud C. Vitamin D supplementation for prevention of cancer in adults. Cochrane Database of Systematic Reviews 2008:CD007469.
- Mittal RD, Manchanda PK, Bhat S, Bid HK. Association of Vitamin-D receptor (Fok-I) gene polymorphism with bladder cancer in an Indian population. BJU Int 2007;99:933-7.
- Raimondi S, Johansson H, Maisonneuve P, Gandini S. Review and meta-analysis on Vitamin D receptor polymorphisms and cancer risk. Carcinogenesis 2009;30:1170-80.
- Eyles D, Brown J, Mackay-Sim A, McGrath J, Feron F. Vitamin D3 and brain development. Neuroscience 2003;118:641-53.
- Sutton AL, Zhang X, Ellison TI, Macdonald PN. The 1,25(OH) 2D3-regulated transcription factor MN1 stimulates Vitamin D receptor-mediated transcription and inhibits osteoblastic cell proliferation. Mol Endocrinol 2005;19:2234-44.
- Arai H, Miyamoto K, Taketani Y, Yamamoto H, Iemori Y, Morita K, et al. AVitamin D receptor gene polymorphism in the translation initiation codon: Effect on protein activity and relation to bone mineral density in Japanese women. J Bone Miner Res 1997;12:915-21.
- Swapna N, Vamsi UM, Usha G, Padma T. Risk conferred by FokI polymorphism of Vitamin D receptor (VDR) gene for essential hypertension. Indian J Hum Genet 2011;17:201-6.
- Jurutka PW, Remus LS, Whitfield GK, Thompson PD, Hsieh JC, Zitzer H, et al. The polymorphic N terminus in human Vitamin D receptor isoforms influences transcriptional activity by modulating interaction with transcription factor IIB. Mol Endocrinol 2000;14:401-20.
- Colin EM, Weel AE, Uitterlinden AG, Buurman CJ, Birkenhäger JC, Pols HA, et al. Consequences of Vitamin D receptor gene polymorphisms for growth inhibition of cultured human peripheral blood mononuclear cells by 1, 25-dihydroxyvitamin D3. Clin Endocrinol (Oxf) 2000;52:211-6.
- Sweeney C, Curtin K, Murtaugh MA, Caan BJ, Potter JD, Slattery ML. Haplotype analysis of common Vitamin D receptor variants and colon and rectal cancers. Cancer Epidemiol Biomarkers Prev 2006;15:744-9.
- Garcion E, Wion-Barbot N, Montero-Menei CN, Berger F, Wion D. New clues about Vitamin D functions in the nervous system. Trends Endocrinol Metab 2002;13:100-5.
- ;McGrath JJ, Féron FP, Burne TH, Mackay-Sim A, Eyles DW. Vitamin D3-implications for brain development. J Steroid Biochem Mol Biol 2004;89-90:557-60.
- Tseng M, Breslow RA, Graubard BI, Ziegler RG. Dairy, calcium, and Vitamin D intakes and prostate cancer risk in the National Health and Nutrition Examination Epidemiologic Follow-up Study cohort. Am J Clin Nutr 2005;81:1147-54.
- Feskanich D, Ma J, Fuchs CS, Kirkner GJ, Hankinson SE, Hollis BW, et al. Plasma Vitamin D metabolites and risk of colorectal cancer in women. Cancer Epidemiol Biomarkers Prev 2004;13:1502-8.
- Qi X, Pramanik R, Wang J, Schultz RM, Maitra RK, Han J, et al. The p38 and JNK pathways cooperate to trans-activate Vitamin D receptor via c-Jun/AP-1 and sensitize human breast cancer cells to Vitamin D(3)-induced growth inhibition. J Biol Chem 2002;277:25884-92.
- Kure S, Nosho K, Baba Y, Irahara N, Shima K, Ng K, et al. Vitamin D receptor expression is associated with PIK3CA and KRAS mutations in colorectal cancer. Cancer Epidemiol Biomarkers Prev 2009;18:2765-72.
- Naveilhan P, Berger F, Haddad K, Barbot N, Benabid AL, Brachet P, et al. Induction of glioma cell death by 1,25(OH) 2 Vitamin D3: Towards an endocrine therapy of brain tumors? J Neurosci Res 1994;37:271-7.
- Baudet C, Chevalier G, Naveilhan P, Binderup L, Brachet P, Wion D. Cytotoxic effects of 1 alpha, 25-dihydroxyvitamin D3 and synthetic Vitamin D3 analogues on a glioma cell line. Cancer Lett 1996;100:3-10.
- Baudet C, Chevalier G, Chassevent A, Canova C, Filmon R, Larra F, et al. 1,25-Dihydroxyvitamin D3 induces programmed cell death in a rat glioma cell line. J Neurosci Res 1996;46:540-50.
- Davoust N, Wion D, Chevalier G, Garabedian M, Brachet P, Couez D. Vitamin D receptor stable transfection restores the susceptibility to 1,25-dihydroxyvitamin D3 cytotoxicity in a rat glioma resistant clone. J Neurosci Res 1998;52:210-9.
- Trouillas P, Honnorat J, Bret P, Jouvet A, Gerard JP. Redifferentiation therapy in brain tumors: Long-lasting complete regression of glioblastomas and an anaplastic astrocytoma under long term 1-alpha-hydroxycholecalciferol. J Neurooncol 2001;51:57-66.
- Neveu I, Naveilhan P, Menaa C, Wion D, Brachet P, Garabédian M. Synthesis of 1,25-dihydroxyvitamin D3 by rat brain macrophages in vitro. J Neurosci Res 1994;38:214-20.
- Toptas B, Kafadar AM, Cacina C, Turan S, Yurdum LM, Yigitbasi N, et al. The Vitamin D receptor (VDR) gene polymorphisms in Turkish brain cancer patients. Biomed Res Int 2013;2013:295791.


 PDF
PDF  Views
Views  Share
Share

