Isolated cerebellar involvement in posterior reversible encephalopathy syndrome in a child with acute lymphoblastic leukemia
CC BY-NC-ND 4.0 · Indian J Med Paediatr Oncol 2011; 32(04): 211-213
DOI: DOI: 10.4103/0971-5851.95143
Abstract
Parieto-occipital region is the most commonly involved site in posterior reversible encephalopathy syndrome (PRES). Cerebellar involvement has been reported with the predominant involvement of posterior cerebral regions, but isolated cerebellar involvement in PRES has been reported only once in English literature. We report here a 7-year-old boy with acute lymphoblastic leukemia who had PRES with isolated cerebellar involvement during induction chemotherapy. He presented with sudden onset headache, vomiting and hypertension followed by seizures, unconsciousness, and involuntary movements. Computed tomography scan revealed bilateral cerebellar hypodensities. He improved within few hours and reversibility of the lesions was documented on magnetic resonance imaging after 2 weeks. Awareness of atypical patterns in distribution of imaging abnormalities is important to recognize PRES more accurately and to avoid unnecessary diagnostic procedures and treatment.
Keywords
Isolated cerebellar involvement - posterior reversible encephalopathy - reversible occipito-parietal leukoencephalopathyPublication History
Article published online:
06 August 2021
© 2011. Indian Society of Medical and Paediatric Oncology. This is an open access article published by Thieme under the terms of the Creative Commons Attribution-NonDerivative-NonCommercial-License, permitting copying and reproduction so long as the original work is given appropriate credit. Contents may not be used for commercial purposes, or adapted, remixed, transformed or built upon. (https://creativecommons.org/licenses/by-nc-nd/4.0/.)
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India
Abstract
Parieto-occipital region is the most commonly involved site in posterior reversible encephalopathy syndrome (PRES). Cerebellar involvement has been reported with the predominant involvement of posterior cerebral regions, but isolated cerebellar involvement in PRES has been reported only once in English literature. We report here a 7-year-old boy with acute lymphoblastic leukemia who had PRES with isolated cerebellar involvement during induction chemotherapy. He presented with sudden onset headache, vomiting and hypertension followed by seizures, unconsciousness, and involuntary movements. Computed tomography scan revealed bilateral cerebellar hypodensities. He improved within few hours and reversibility of the lesions was documented on magnetic resonance imaging after 2 weeks. Awareness of atypical patterns in distribution of imaging abnormalities is important to recognize PRES more accurately and to avoid unnecessary diagnostic procedures and treatment.
INTRODUCTION
Posterior reversible encephalopathy syndrome (PRES) is a reversible clinicoradiological syndrome characterized clinically by acute onset headache, altered alertness, seizures, vomiting, and abnormalities of visual perception, and neuroradiologically by edema predominantly involving the parieto-occipital regions. Cerebellar involvement has been reported with the predominant involvement of posterior cerebral regions, but review of published English literature revealed only one case report with isolated cerebellar involvement in PRES.[1] We report here a case of PRES with isolated cerebellar involvement in a child with acute lymphoblastic leukemia (ALL) during induction chemotherapy.
CASE REPORT
A 7-years-old boy presented with slowly progressive bilateral cervical lymphadenopathy of few months’ duration. There were no constitutional symptoms. He had pallor, nontender generalized lymphadenopathy, and splenomegaly. Biopsy from cervical lymph node revealed lymphoblastic lymphoma. Laboratory investigations at admission revealed pancytopenia with 20% blasts in peripheral blood. Bone marrow aspiration revealed 65% blasts. Immunophenotyping of bone marrow aspirate suggested T-cell ALL. Central nervous system was uninvolved on cerebrospinal fluid examination. Induction chemotherapy was initiated as per MCP 841 protocol which includes prednisolone, 40 mg/m2 for 29 days; L-asparaginase, 6 000 IU/m2 on alternate days for 10 doses; vincristine, 1.4 mg/m2 weekly for 5 doses; daunorubicin, 30 mg/m2 at day 8, 15, and 29; and weekly intrathecal injections of methotrexate for 4 weeks. After documentation of febrile neutropenia on 14th day of induction, piperacillin-tazobactam and amikacin were initiated. He responded well and became afebrile within 24 hours of initiation of antibiotics.
On 19th day of induction, he complained of sudden onset headache and projectile vomiting. He was lethargic but well oriented and did not have signs of meningitis, abnormal movements, or visual disturbances. Fundus examination was normal bilaterally. He was hypertensive initially (blood pressure, 150/110 mmHg; >99th centile), but subsequent records were within normal range. A few hours later, the patient had two episodes of left focal tonic-clonic seizures at one-hour interval which were managed with midazolam and phenytoin. The seizure was followed by loss of consciousness and some involuntary movements. Computed tomography (CT) of brain revealed diffuse hypodensity involving bilateral cerebellar hemispheres [Figure 1a]. Patient became fully conscious after about 3 to 4 hours without any neurological deficit. Follow-up cranial magnetic resonance imaging (MRI) examination performed 2 weeks after the episode showed almost complete resolution of cerebellar lesions [Figure 1b]. He achieved remission after induction and received two cycles of high-dose cytarabine (I2A) and repeat induction (RI1) cycle. He did not develop any neurological symptom during subsequent chemotherapy, but unfortunately succumbed to infective endocarditis after the third cycle of chemotherapy.
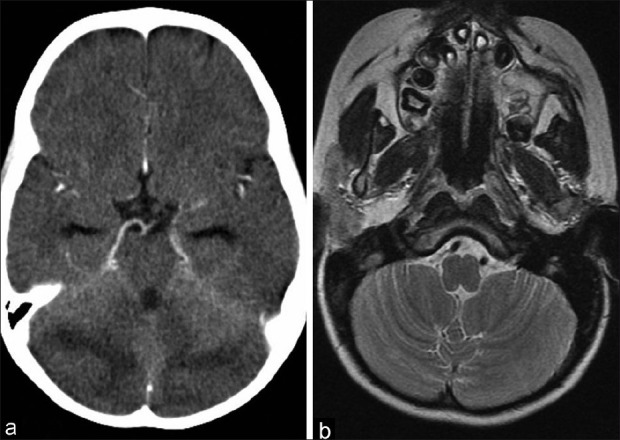
| Figure 1:(a) CT scan brain showing bilateral cerebellar edema; (b) MRI after 2 weeks showing complete resolution of cerebellar edema
DISCUSSION
The reversible syndrome of altered mental status, headache, seizures, visual disturbances, and hypertension has been described in literature with different terminologies like hypertensive encephalopathy, reversible posterior leukoencephalopathy, and reversible occipitoparietal leukoencephalopathy.[2]
This syndrome has been recognized without hypertension or with only mild increase in blood pressure; there is usually no accompanying destructive process of the white matter; and neuroradiologically, the involvement is not strictly restricted to parieto-occipital regions. Hence, PRES is more acceptable and popular terminology.[3,4]
PRES is seen exclusively in the setting of a significant systemic process or condition, including sudden hypertension, bone marrow or solid organ transplantation (specially on cyclosporine immunosuppression), infection/sepsis/shock, toxemia of pregnancy, autoimmune disease, and during cancer chemotherapy (e.g., l-asparaginase, prednisolone, high-dose cytarabine, etc.). A case series from India revealed induction chemotherapy, hypertension, high-dose cytarabine, and cyclosporine as the triggers for PRES in 13 leukemic patients.[5]
Cerebral hypoperfusion and cytotoxic edema secondary to underlying systemic condition or drugs seems to be the principle pathophysiologic process leading to development of encephalopathy. Hypertension may have a modulating effect to this process.[3] An obvious risk factor in our patient was induction chemotherapy for ALL, but he also had one record of sudden onset hypertension at the time of neurotoxicity, which might have modulating effect. Furthermore, despite receiving high-dose cytarabine and repeat induction cycle subsequently, PRES did not recur, probably due to absence of hypertension during subsequent therapy.
Although MRI is the most sensitive imaging test to diagnose PRES, it is not necessary for the diagnosis if CT is suggestive. CT usually shows bilateral hypodensities in the posterior white matter, with some involvement of the overlying cortex. On MRI, these lesions appear hyperintense on T2-weighted images, and are usually hypointense or isointense on diffusion-weighted images, with an increase of the apparent diffusion coefficient, indicating vasogenic edema. Mckinney et al. reported two cases of completely unilateral involvement in their series, which is difficult to distinguish from infarction.[6] The calcarine and paramedian occipital-lobe structures are usually spared, a fact that distinguishes PRES from bilateral infarction of the posterior-cerebral-artery territory.[4] The posterior part of the brain is more frequently involved due to poor sympathetic innervation of the vertebrobasilar system.
Fugate et al. found the parieto-occipital regions as the most commonly involved site (94%) on MRI in a large prospective study, followed by the frontal lobe (77%), temporal lobe (64%), and cerebellum (53%). Cerebellar involvement was significantly more frequent in patients with a history of autoimmunity, and patients with sepsis were more likely to have cortical involvement.[7] Although parieto-occipital involvement was found in 99% of the cases, McKinney et al. noted higher incidence of atypical distributions (posterior frontal, 78.9%; temporal, 68.4%; thalamus, 30.3%; cerebellum, 34.2%; brainstem, 18.4%; and basal ganglia, 11.8%) than commonly perceived. Only one patient lacked parieto-occipital edema, but this patient had severe brainstem, thalamic, and deep white matter edema.[6] “Atypical” cases with only frontal, temporal, or brain stem lesions have been reported in literature. Only one case of PRES with isolated cerebellar involvement has been reported in the English literature, ours being the second.[1]
Although the reversibility of the clinicoradiological features is most characteristic, it should be noted that prolonged seizures, hypertension, or both may result in permanent neurologic deficits and cerebral infarction. Hence, PRES must be managed with good supportive care including antihypertensive and antiepileptic medications to prevent any irreversible damage. A follow-up scan in a period of 1 to 2 weeks is needed to document the reversibility.[8]
CONCLUSION
Awareness of atypical patterns and variations in distribution of imaging abnormalities is important to recognize PRES more accurately, to avoid expensive or potentially invasive work-ups for other primary cerebral diseases.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- Soysal DD, Caliskan M, Aydin K, Nayir A, Karaböcüoglu M, Citak A, et al. Isolated cerebellar involvement in a case of posterior reversible leukoencephalopathy. Clin Radiol 2006;61:983-6.
- Pavlakis SG, Frank Y, Chusid R. Hypertensive encephalopathy, reversible occipitoparietal encephalopathy, or reversible posterior leukoencephalopathy: Three names for an old syndrome. J Child Neurol 1999;14:277-81.
- Bartynski WS. Posterior reversible encephalopathy syndrome, part 2: Controversies surrounding pathophysiology of vasogenic edema. AJNR Am J Neuroradiol 2008;29:1043-9.
- Hinchey J, Chaves C, Appignani B, Breen J, Pao L, Wang A, et al. A reversible posterior leukoencephalopathy syndrome. N Engl J Med 1996;334:494-500.
- Dongre A, Gulia S, Arora B, Kurkure PA, Banavali SD. Posterior reversible encephalopathy syndrome (PRES) in pediatric cancer patients. PHOCON 2009/OP1 [Abstract]. Available from: http://www.phoindia.org/pdf/all-abstracts-PHOCON-2009-chandigarh.pdf [Last accessed on 2010 Dec 26].
- McKinney AM, Short J, Truwit CL, McKinney ZJ, Kozak OS, SantaCruz KS, et al. Posterior reversible encephalopathy syndrome: Incidence of atypical regions of involvement and imaging findings. AJR Am J Roentgenol 2007;189:904-12.
- Fugate JE, Claassen DO, Cloft HJ, Kallmes DF, Kozak OS, Rabinstein AA. Posterior reversible encephalopathy syndrome: Associated clinical and radiologic findings. Mayo Clin Proc 2010;85:427-32.
- Prasad N, Gulati S, Gupta RK, Kumar R, Sharma K, Sharma RK. Is reversible posterior leukoencephalopathy with severe hypertension completely reversible in all patients? Pediatr Nephrol 2003;18:1161-6.

| Figure 1:(a) CT scan brain showing bilateral cerebellar edema; (b) MRI after 2 weeks showing complete resolution of cerebellar edema
References
- Soysal DD, Caliskan M, Aydin K, Nayir A, Karaböcüoglu M, Citak A, et al. Isolated cerebellar involvement in a case of posterior reversible leukoencephalopathy. Clin Radiol 2006;61:983-6.
- Pavlakis SG, Frank Y, Chusid R. Hypertensive encephalopathy, reversible occipitoparietal encephalopathy, or reversible posterior leukoencephalopathy: Three names for an old syndrome. J Child Neurol 1999;14:277-81.
- Bartynski WS. Posterior reversible encephalopathy syndrome, part 2: Controversies surrounding pathophysiology of vasogenic edema. AJNR Am J Neuroradiol 2008;29:1043-9.
- Hinchey J, Chaves C, Appignani B, Breen J, Pao L, Wang A, et al. A reversible posterior leukoencephalopathy syndrome. N Engl J Med 1996;334:494-500.
- Dongre A, Gulia S, Arora B, Kurkure PA, Banavali SD. Posterior reversible encephalopathy syndrome (PRES) in pediatric cancer patients. PHOCON 2009/OP1 [Abstract]. Available from: http://www.phoindia.org/pdf/all-abstracts-PHOCON-2009-chandigarh.pdf [Last accessed on 2010 Dec 26].
- McKinney AM, Short J, Truwit CL, McKinney ZJ, Kozak OS, SantaCruz KS, et al. Posterior reversible encephalopathy syndrome: Incidence of atypical regions of involvement and imaging findings. AJR Am J Roentgenol 2007;189:904-12.
- Fugate JE, Claassen DO, Cloft HJ, Kallmes DF, Kozak OS, Rabinstein AA. Posterior reversible encephalopathy syndrome: Associated clinical and radiologic findings. Mayo Clin Proc 2010;85:427-32.
- Prasad N, Gulati S, Gupta RK, Kumar R, Sharma K, Sharma RK. Is reversible posterior leukoencephalopathy with severe hypertension completely reversible in all patients? Pediatr Nephrol 2003;18:1161-6.


 PDF
PDF  Views
Views  Share
Share

