Isolated Primary non?Hodgkin’s Lymphoma of the Esophagus
CC BY-NC-ND 4.0 · Indian J Med Paediatr Oncol 2018; 39(02): 244-246
DOI: DOI: 10.4103/ijmpo.ijmpo_131_17
Abstract
Isolated primary esophageal lymphoma, defined as a lymphoma developing in the esophageal wall, is a distinctly rare presentation and accounts for <0>
Publication History
Article published online:
23 June 2021
© 2018. Indian Society of Medical and Paediatric Oncology. This is an open access article published by Thieme under the terms of the Creative Commons Attribution-NonDerivative-NonCommercial-License, permitting copying and reproduction so long as the original work is given appropriate credit. Contents may not be used for commercial purposes, or adapted, remixed, transformed or built upon. (https://creativecommons.org/licenses/by-nc-nd/4.0/.)
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India
Abstract
Isolated primary esophageal lymphoma, defined as a lymphoma developing in the esophageal wall, is a distinctly rare presentation and accounts for <1>
Introduction
Non-Hodgkin's lymphoma (NHL) is most commonly a disease primarily involving lymph nodes; however, it has a well-known tendency to involve extranodal organs as its primary site. The gastrointestinal (GI) tract is the most common extranodal site of NHL, accounting for 5%–20% of all cases,[1] with the stomach affected in approximately 50%, the small bowel or ileocecal valve in approximately 33%, and the large bowel in approximately 10%. Esophageal lymphoma accounts for <1 href="https://www.thieme-connect.com/products/ejournals/html/10.4103/ijmpo.ijmpo_131_17#JR_2" xss=removed>2] In a study of 79 cases of isolated lymphomas of the GI tract in HIV-seronegative patients, sites of involvement were the stomach (55%), small intestine (31%), large intestine (11%), and esophagus (1%). Lymphomas account for <1 href="https://www.thieme-connect.com/products/ejournals/html/10.4103/ijmpo.ijmpo_131_17#JR_3" xss=removed>3] We report a case of histopathologically confirmed primary esophageal NHL of diffuse large B-cell type (DLBCL), describe its clinicoradiological features, and review the literature.
Case Report
A 60-year-old man presented to us with recent onset progressive dysphagia for solids over the past 6 months. There was no history suggestive of gastroesophageal reflux or esophagitis before initiation of his present symptoms. His physical examination was normal without enlarged lymph nodes or palpable hepatosplenomegaly. Routine hematological and biochemical investigations were normal. Esophagogastroduodenoscopy showed a circumferential ulceroproliferative friable tumor from 30 to 35 cm with luminal narrowing. The remaining part of the esophagus, stomach, and duodenum were normal. The biopsy showed malignant round cells arranged in a diffuse pattern, suggestive of a NHL [Figure 1]a and [Figure 1]b. This was further confirmed by immunohistochemistry which showed diffuse positivity of tumor cells for leukocyte common antigen [Figure 1]c and CD20 [Figure 1]d and negativity for CD3 antigen. Mib-1/Ki-67 (proliferation marker) labeling index highlighted 98% tumor cell nuclei. Cytokeratin, epithelial membrane antigen, and CD138 were negative. Positron emission tomography (PET) scan [Figure 2]a and [Figure 2]b revealed 18-F fluorodeoxyglucose-avid circumferential wall thickening of the mid and lower third of the esophagus for a segment of 10 cm with hypermetabolic pretracheal, precarinal, and bilateral hilar lymph nodes. A bone marrow biopsy was done which did not show involvement by NHL. Based on a final diagnosis of isolated esophageal NHL of DLBCL type, and the multidisciplinary tumor board decision, we treated the patient with chemoradiation with six cycles of chemotherapy, including cyclophosphamide, etoposide, vincristine, and prednisolone, along with involved field radiotherapy) in view of the bulky primary disease. Posttreatment response assessment PET [Figure 2]c and [Figure 2]d revealed complete metabolic and morphological response of the primary and aortopulmonary lymph nodes. Endoscopic mapping after completion of treatment showed only posttreatment changes in the form of mucosal tags with scarring and pseudodiverticula. The patient is symptom and disease free on a 3-year follow-up.

| Figure.1(a) Tumor underlying esophageal squamous mucosa.(b) Malignant tumor composed of round cells arranged in a diffuse pattern. (c) Tumor cells diffusely expressing leukocyte common antigen. (d) Diffuse CD20 positivity within tumor cells
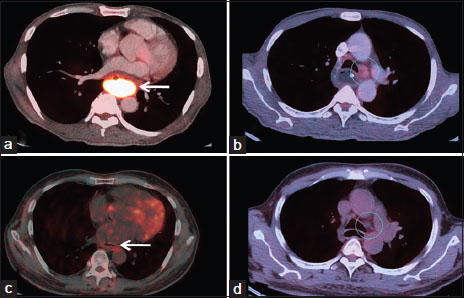
| Figure.2Pre- and post treatment 18-F fluorodeoxyglucose positron emission tomography-contrast-enhanced computed tomography study in a patient diagnosed of esophageal Non-Hodgkin’s Lymphoma. Axial-fused positron emission tomography-computed tomography images show the bulky 18-F fluorodeoxyglucose avid esophageal mass (arrow in 2a) and aortopulmonary lymph node (circle in 2b). Post treatment positron emission tomography-contrast-enhanced computed tomography study showing complete metabolic and morphological response of the circumferential thickening in the middle and lower third esophagus (arrow in 2c) and also the aortopulmonary nodes (circle in 2d)
Discussion
Epithelial tumors, namely squamous cell carcinoma and adenocarcinoma, are the most common types of esophageal cancer. Nonepithelial malignancies comprise only about 0.5% of all primary esophageal neoplasms. Primary lymphoma of the GI tract accounts for about 5%–20% of all cases of lymphoma. Isolated NHL of the esophagus is an extremely uncommon occurrence, accounting for <1 href="https://www.thieme-connect.com/products/ejournals/html/10.4103/ijmpo.ijmpo_131_17#JR_4" xss=removed>4] Furthermore, isolated primary lymphoma of the esophagus without an extraesophageal location is extremely rare and only 20 such cases have been reported in literature to date.[5],[6]
The etiology of the disease is unknown, with the role of Epstein–Barr virus being controversial. It has been noticed that it is most common in immune compromised patients, with HIV infection as a probable risk factor. Dysphagia is the most common symptom at presentation with less common symptoms being odynophagia, fever, and weight loss. The age at presentation of the disease is highly variable. Endoscopic and radiological findings of esophageal lymphoma are nonspecific and nondiagnostic. The diverse spectrum of endoscopic/radiological patterns that have been described for esophageal lymphoma include stricture, ulcerated mass, multiple submucosal nodules, varicoid pattern, achalasia-like pattern, progressive aneurysmal dilatation, and tracheoesophageal fistula formation. Computed tomography (CT) scan is valuable for the evaluation of the extraluminal component of an esophageal mass, its mediastinal extension, fistula formation, and status of the lymph nodes; therefore, it has a role in disease staging, assisting in stratification of various available treatments, evaluating treatment responses, monitoring patient progress, as well as detection of any relapses. PET-CT scanning has emerged as an indispensable tool in the staging and follow-up of patients with extranodal involvement in lymphoma. PET-CT has also significantly increased the detection of indolent lesions that were undetected by conventional cross-sectional imaging.[7]
Dawson's criteria [8] to identify primary GI lymphoma include nonpalpable superficial lymphadenopathy, no splenic involvement, and no enlargement of mediastinal or hilar lymph nodes. Our patient fit Dawson's criteria fully, except that he also had enlarged mediastinal nodes. The application of Dawson's criteria for the diagnosis of primary GI lymphoma may not hold true for the esophagus. Modifications as exclusion of mediastinal lymphadenopathy in the criteria for primary esophageal lymphoma have been suggested, because the esophagus is a mediastinal structure with an extensive lymphatic network which results in wide lymph node basins. Therefore, based on the predominant lesion being in the esophagus with no involvement of liver, spleen, and peripheral lymph nodes, we did not consider the absence of mediastinal lymphadenopathy as strict criteria for a diagnosis of primary esophageal lymphoma; the positive endoluminal biopsy in the absence of nodal disease infiltrating the esophageal wall confirmed the primary origin in the esophagus.
Conclusion
Primary NHL of the esophagus is an extremely rare esophageal neoplasm. Imaging modalities are nonspecific, thus posing a diagnostic dilemma. Endoscopic biopsy is the gold standard to confirm the diagnosis. Accurate histopathological diagnosis supplemented with necessary immunohistochemical stains helps in accurate diagnosis; complete staging work-up using PET-CT scanning aids in treatment decision and prognostication. Appropriate chemotherapy with radiotherapy may offer a significant chance of long-term survival.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Conflict of Interest
There are no conflicts of interest.
References
- Herrmann R, Panahon AM, Barcos MP, Walsh D, Stutzman L. Gastrointestinal involvement in non-Hodgkin's lymphoma. Cancer 1980; 46: 215-22
- Weeratunge CN, Bolivar HH, Anstead GM, Lu DH. Primary esophageal lymphoma: A diagnostic challenge in acquired immunodeficiency syndrome – Two case reports and review. South Med J 2004; 97: 383-7
- Allen AW, Donaldson G, Sniffen RC, Goodale FJr. Primary malignant lymphoma of the gastro-intestinal tract. Ann Surg 1954; 140: 428-38
- Okerbloom JA, Armitage JO, Zetterman R, Linder J. Esophageal involvement by non-Hodgkin's lymphoma. Am J Med 1984; 77: 359-61
- Golioto M, McGrath K. Primary lymphoma of the esophagus in a chronically immunosuppressed patient with hepatitis C infection: Case report and review of the literature. Am J Med Sci 2001; 321: 203-5
- Gupta S, Pant GC, Gupta S. A clinicopathological study of primary gastrointestinal lymphoma. J Surg Oncol 1981; 16: 49-58
- Paes FM, Kalkanis DG, Sideras PA, Serafini AN. FDG PET/CT of extranodal involvement in non-Hodgkin lymphoma and Hodgkin disease. Radiographics 2010; 30: 269-91
- Dawson IM, Cornes JS, Morson BC. Primary malignant lymphoid tumours of the intestinal tract.Report of 37 cases with a study of factors influencing prognosis. Br J Surg 1961; 49: 80-9
Address for correspondence
Publication History
Article published online:
23 June 2021
© 2018. Indian Society of Medical and Paediatric Oncology. This is an open access article published by Thieme under the terms of the Creative Commons Attribution-NonDerivative-NonCommercial-License, permitting copying and reproduction so long as the original work is given appropriate credit. Contents may not be used for commercial purposes, or adapted, remixed, transformed or built upon. (https://creativecommons.org/licenses/by-nc-nd/4.0/.)
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2,
Noida-201301 UP, India

| Figure.1(a) Tumor underlying esophageal squamous mucosa.(b) Malignant tumor composed of round cells arranged in a diffuse pattern. (c) Tumor cells diffusely expressing leukocyte common antigen. (d) Diffuse CD20 positivity within tumor cells

| Figure.2Pre- and post treatment 18-F fluorodeoxyglucose positron emission tomography-contrast-enhanced computed tomography study in a patient diagnosed of esophageal Non-Hodgkin’s Lymphoma. Axial-fused positron emission tomography-computed tomography images show the bulky 18-F fluorodeoxyglucose avid esophageal mass (arrow in 2a) and aortopulmonary lymph node (circle in 2b). Post treatment positron emission tomography-contrast-enhanced computed tomography study showing complete metabolic and morphological response of the circumferential thickening in the middle and lower third esophagus (arrow in 2c) and also the aortopulmonary nodes (circle in 2d)
References
- Herrmann R, Panahon AM, Barcos MP, Walsh D, Stutzman L. Gastrointestinal involvement in non-Hodgkin's lymphoma. Cancer 1980; 46: 215-22
- Weeratunge CN, Bolivar HH, Anstead GM, Lu DH. Primary esophageal lymphoma: A diagnostic challenge in acquired immunodeficiency syndrome – Two case reports and review. South Med J 2004; 97: 383-7
- Allen AW, Donaldson G, Sniffen RC, Goodale FJr. Primary malignant lymphoma of the gastro-intestinal tract. Ann Surg 1954; 140: 428-38
- Okerbloom JA, Armitage JO, Zetterman R, Linder J. Esophageal involvement by non-Hodgkin's lymphoma. Am J Med 1984; 77: 359-61
- Golioto M, McGrath K. Primary lymphoma of the esophagus in a chronically immunosuppressed patient with hepatitis C infection: Case report and review of the literature. Am J Med Sci 2001; 321: 203-5
- Gupta S, Pant GC, Gupta S. A clinicopathological study of primary gastrointestinal lymphoma. J Surg Oncol 1981; 16: 49-58
- Paes FM, Kalkanis DG, Sideras PA, Serafini AN. FDG PET/CT of extranodal involvement in non-Hodgkin lymphoma and Hodgkin disease. Radiographics 2010; 30: 269-91
- Dawson IM, Cornes JS, Morson BC. Primary malignant lymphoid tumours of the intestinal tract.Report of 37 cases with a study of factors influencing prognosis. Br J Surg 1961; 49: 80-9


 PDF
PDF  Views
Views  Share
Share

