Letrozole and Palbociclib in Advanced Breast Cancer: Outcome from Cancer Institute, Chennai
CC BY-NC-ND 4.0 · Indian J Med Paediatr Oncol 2020; 41(02): 182-186
DOI: DOI: 10.4103/ijmpo.ijmpo_156_19
Abstract
Background: Cyclin-dependent kinase 4/6 inhibitor addition to hormonal therapy has shown to improve the survival of hormone receptor (HR)-positive, HER2-negative advanced breast cancer (ABC). Methods: We retrospectively analyzed untreated patients with HR-positive, HER2-negative ABC, who received letrozole and palbociclib at the Cancer Institute, Chennai, from October 2017 to January 2019. Results: A total of 24 patients were included in this study. The median progression-free survival (PFS) was 18 months, and the median overall survival (OS) had not reached. The 1-year PFS and OS were 73.7% and 89.2%, respectively. The common toxicities were neutropenia and fatigue but none of the patients had febrile neutropenia. Conclusion: Letrozole-Palbociclib is effective with manageable toxicity as the first-line treatment for HR-positive, HER2-negative ABC.
Publication History
Received: 22 July 2019
Accepted: 02 January 2020
Article published online:
23 May 2021
© 2020. Indian Society of Medical and Paediatric Oncology. This is an open access article published by Thieme under the terms of the Creative Commons Attribution-NonDerivative-NonCommercial-License, permitting copying and reproduction so long as the original work is given appropriate credit. Contents may not be used for commercial purposes, or adapted, remixed, transformed or built upon. (https://creativecommons.org/licenses/by-nc-nd/4.0/.)
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India
Abstract
Background: Cyclin-dependent kinase 4/6 inhibitor addition to hormonal therapy has shown to improve the survival of hormone receptor (HR)-positive, HER2-negative advanced breast cancer (ABC). Methods: We retrospectively analyzed untreated patients with HR-positive, HER2-negative ABC, who received letrozole and palbociclib at the Cancer Institute, Chennai, from October 2017 to January 2019. Results: A total of 24 patients were included in this study. The median progression-free survival (PFS) was 18 months, and the median overall survival (OS) had not reached. The 1-year PFS and OS were 73.7% and 89.2%, respectively. The common toxicities were neutropenia and fatigue but none of the patients had febrile neutropenia. Conclusion: Letrozole-Palbociclib is effective with manageable toxicity as the first-line treatment for HR-positive, HER2-negative ABC.
Keywords
Advanced breast cancer - letrozole - palbociclibIntroduction
According to the GLOBOCAN 2018, breast cancer is the most common cancer in Indian women constituting 28% of all cancers.[1] In India, 21% of patients with breast cancer have metastasis at the time of presentation.[2] The first-line treatment for hormone receptor (HR)-positive and HER2-negative advanced breast cancer (ABC) without visceral crisis is hormonal therapy.[3] However, patients usually progress after a few months due to endocrine resistance.[4] The endocrine resistance can be overcome by the addition of mammalian target of rapamycin or cyclin-dependent kinase (CDK) inhibitors along with hormonal therapy.[5]
Flavopiridol ( first-generation CDK inhibitor) is a synthetic flavone purified from Dysoxylum binectariferum, a plant indigenous to India and used in the Indian traditional medicine.[6] However, it was nonselective and had significant toxicity in early clinical trials.[7] The second-generation CDK inhibitors targeting CDK 4/6 pathway have shown activity and a manageable toxicity profile.[8] The addition of CDK 4/6 inhibitors such as palbociclib,[9] ribociclib,[10] and abemaciclib[11] has shown to improve the progression-free survival (PFS) by 1 year as compared to only hormonal therapy. Recently, it was shown that ribociclib with hormonal therapy improved the overall survival (OS) by 24% as compared to only hormonal therapy.[12] The advantages of CDK 4/6 inhibitors are oral therapy and manageable toxicity profile. Palbociclib was approved by the United States Food and Drug Administration in March 2017 as frontline therapy for HR-positive, HER2-negative ABC and was launched in India in October 2017. This initial report is our experience of the efficacy and toxicity of letrozole-palbociclib as the initial treatment in ABC.
Methods
Data were obtained and analyzed from individual case records of patients with ABC who received letrozole and palbociclib from October 2017 to January 2019 at the Cancer Institute, Chennai. Patients included in this analysis were previously untreated, postmenopausal, HR-positive (estrogen receptor [ER] or progesterone receptor [PR] >1%), HER2-negative ABC who received the first-line therapy with tablet letrozole-palbociclib. Patients who had progression while on hormonal therapy and those who received palbociclib as the second-line therapy were excluded from the analysis.
The investigations performed at diagnosis were contrast-enhanced computed tomography chest and abdomen/pelvis with a bone scan or positron emission tomography–computed tomography scan. The schedule of treatment was tablet letrozole 2.5 mg once daily and capsule palbociclib 125 mg, 3 out of 4 weeks in a 28-day cycle. Patients had a complete hemogram before starting treatment, D14 of the first two cycles and before the start of each cycle. Adverse effects were captured according to the Common Terminology Criteria for Adverse Events (CTCAE) version 4.0 (National Cancer Institute, Bethesda, Maryland, United States). Patients with Grade 3/4 toxicity due to palbociclib had a reduced dose of 100 mg or 75 mg daily in a step-wise fashion. Clinical breast examination was performed before each cycle and imaging once in 2–3 months and when clinically indicated.
Statistical methods
Descriptive statistics were used for the baseline characteristics. PFS was calculated from the date of starting letrozole and palbociclib until the progression or death. OS was calculated from the date of starting letrozole and palbociclib until death. The Kaplan–Meier method was used to obtain estimates of median PFS, with corresponding two-sided 95% confidence intervals (CIs). The log-rank test was used for univariate analysis, and P < 0.05 was considered statistically significant. The analysis was done using SPSS version 23, IBM Corporation, New York, United States.
Results
A total of 24 patients were included in this analysis, with a median duration of follow-up of 13 months (range: 5–28 months). The median age was 56 years (range: 25–71 years). Sixty-six percent of patients had a comorbid illness (diabetes mellitus in 33% and systemic hypertension in 33%). The histology was infiltrating ductal carcinoma in all the patients. The differentiation was Grade 1 (8%), Grade 2 (58%), and Grade 3 (33%). The median ER, PR, and Ki-67 were 90% (range: 40%–90%), 45% (range: 0%–90%), and 15% (range: 5%–80%), respectively. T-staging was T1 (4%), T2 (8%), T3 (16%), and T4 (54%) disease. The median tumor size was 6 cm (range: 2–12 cm). The nodal stage was N1 (29%), N2 (41%), and N3 (16%). The sites of metastasis were bone (70%), lung (54%), liver (25%), and lymph nodes (16%). Metastatic disease confined only to bones was present in 9 patients (37.5%) [Table 1].
Table 1 Baseline characteristics (n=24)
Among 24 patients, 21 patients had evaluable disease [Table 2]. At a median follow-up of 13 months, there were 19% complete response (CR, n = 4), 19% (PR, n = 4), 24% stable disease (SD, n = 5), and 38% progressive disease (PD, n = 8). Among the four patients who achieved CR, there was one patient with extensive disease involving the brain, liver, bone, and nodes who achieved a complete metabolic response.
Response (n=18)
|
Response |
n (%) |
|---|---|
|
Complete remission |
4 (19) |
|
Partial remission |
4 (19) |
|
Stable disease |
5 (24) |
|
Progressive disease |
8 (38) |
Among the patients who had progressive disease (n = 8), 37.5% (n = 3) had systemic progression, 37.5% (n = 3) had locoregional progression, and 25% (n = 2) had both systemic and locoregional progression. The median PFS was 18 months (95% CI: 15–20 months), and the median OS was not reached. The 1-year PFS and OS were 73.7% and 89.2%, respectively [Figure 1] and [Figure 2]. Among the patients who had progressive disease (n = 8), three were switched to fulvestrant, two to tamoxifen, one to exemestane, and two were unwilling for further treatment and opted for the best supportive care.
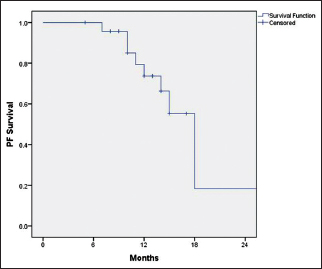
| Figure 1: Progression-free survival
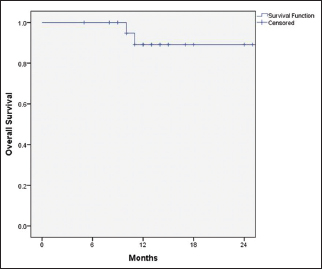
| Figure 2: Overall survival
Univariate analysis [Table 3] with age, Eastern Cooperative Oncology Group performance status (PS), type (de novo vs. recurrent disease), sites of metastasis, grade, Ki-67, and number of sites of metastasis and palbociclib dose reduction were correlated with PFS. Patients with >3 sites of metastasis had a higher risk of progression (Hazard ratio: 8.7; 95% CI: 1.2–63.4; P = 0.03).
Table 3 Univariate analysis of factors predicting progression-free survival
The nadir white blood cell count and platelet count were 2000 cells/mm3 and 50,000/μl, respectively. However, it was not clinically significant, as none of them had febrile neutropenia or bleeding manifestations. None of the patients had Grade 4 toxicities. Grade 3 leukopenia was seen in two patients and thrombocytopenia in one patient. Grade 1 or 2 side effects were leukopenia 83%, thrombocytopenia in 25%, and fatigue in 33%. About 29% of patients required dose reduction due to fatigue or mucositis.
Discussion
The striking difference between our patients and the Western population[13] is that majority of our patients have locally advanced disease (T4/T4 – 70%; N2/N3 – 57%) at presentation. There are no published studies form India on hormonal therapy with palbociclib except an abstract that showed a response rate of 41%.[14]
PALOMA-2 trial had 23% of patients with metastasis confined to the bones as compared to our study (37%). Patients with metastasis confined to bones have improved survival as compared to those with visceral disease.[15] The response rate was 55% in the PALOMA-2 trial as compared to 38% in this study. Complete response was seen in four patients (19%), and none of them had progressed at the time of follow-up. All the CDK 4/6 inhibitors trials excluded patients with brain metastasis. We had an unusual patient with brain metastasis who achieved a complete metabolic remission with letrozole and palbociclib. A recent study reported that CDK 4/6 inhibitors in combination with stereotactic radiation for brain prolonged survival.[16]
The median PFS in our study was 18 months as compared to the PALOMA-2 trial where it was 24.9 months. This reflects the fact that real-world outcome is usually inferior to that reported in clinical trials.[17] The reasons could be due to the inclusion of all comers, decreased compliance with drug intake, and shorter follow-up.
Meta-analysis has shown that hormonal therapy with CDK 4/6 inhibitor significantly improves PFS as compared to those taking only hormonal therapy or chemotherapy in HR-positive, HER2-negative ABC.[18] There is an ongoing phase 3 randomized controlled trial comparing ribociclib and endocrine therapy versus chemotherapy as the first-line therapy in HR-positive, HER2-negative ABC in visceral crisis.
The febrile neutropenia was uncommon in the PALOMA trial (1.8%) and there was none in this study.[9] None of our patients had bleeding manifestations due to thrombocytopenia. Twenty-four percent of patients required dose reductions due to fatigue or mucositis. However, none of them required drug discontinuation. Currently, there is no standard therapy for patients who progress on letrozole and palbociclib. The options can be alpelisib and fulvestrant in PIK3CA-mutated tumors,[19] abemaciclib,[20] exemestane everolimus,[21] fulvestrant, or chemotherapy. Enrollment in clinical trials could be an option in view of lack of standard treatment.
The subset analysis from all the three CDK 4/6 inhibitors showed that all the subgroups (PS 0 vs. 1, visceral vs. bone metastasis, de novo vs. recurrent, chemotherapy naïve vs. treated, type of endocrine therapy) have uniformly derived benefit from the addition of CDK 4/6 inhibitors to hormonal therapy. The potential biomarkers are CCND1 amplification, loss of p16, CDK 4 and 6 levels, Rb expression, and Ki-67.[22] Overexpression of TK1 and CDK9 can lead to resistance to CDK 4/6 inhibitors.[23] Biomarkers analysis has shown that both luminal A and luminal B tumors respond to letrozole with palbociclib and lower PD1 levels associated with greater benefit.[24] The limitations of this study are single-institutional study, nonrandomized design, small sample size, and shorter follow-up.
Conclusion
Letrozole-palbociclib is effective with manageable toxicity as the first-line treatment for HR-positive, HER2-negative ABC. Visceral metastasis or large primary tumor is not an indication to start the chemotherapy in HR-positive, HER2-negative ABC.
Conflict of Interest
There are no conflicts of interest.
Acknowledgment
We would like to thank Pfizer for supporting free palbociclib to patients below the poverty line. Furthermore, other patients were supported free palbociclib after the completion of ten cycles.
References
- Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 Countries. CA Cancer J Clin 2018; 68: 394-424
- Nair N, Shet T, Parmar V, Havaldar R, Gupta S, Budrukkar A. et al. Breast cancer in a tertiary cancer center in India – An audit, with outcome analysis. Indian J Cancer 2018; 55: 16-22
- Matutino A, Joy AA, Brezden-Masley C, Chia S, Verma S. Hormone receptor-positive, HER2-negative metastatic breast cancer: Redrawing the lines. Curr Oncol 2018; 25: S131-41
- Osborne CK, Schiff R. Mechanisms of endocrine resistance in breast cancer. Annu Rev Med 2011; 62: 233-47
- AlFakeeh A, Brezden-Masley C. Overcoming endocrine resistance in hormone receptor-positive breast cancer. Curr Oncol 2018; 25: S18-27
- Sedlacek H, Czech J, Naik R, Kaur G, Worland P, Losiewicz M. et al. Flavopiridol (L86 8275 NSC 649890), a new kinase inhibitor for tumor therapy. Int J Oncol 1996; 9: 1143-68
- Tan AR, Yang X, Berman A, Zhai S, Sparreboom A, Parr AL. et al. Phase I trial of the cyclin-dependent kinase inhibitor flavopiridol in combination with docetaxel in patients with metastatic breast cancer. Clin Cancer Res 2004; 10: 5038-47
- DiPippo AJ, Patel NK, Barnett CM. Cyclin-dependent kinase inhibitors for the treatment of breast cancer: Past, present, and future. Pharmacotherapy 2016; 36: 652-67
- Finn RS, Martin M, Rugo HS, Jones S, Im SA, Gelmon K. et al. Palbociclib and letrozole in advanced breast cancer. N Engl J Med 2016; 375: 1925-36
- Hortobagyi GN, Stemmer SM, Burris HA, Yap YS, Sonke GS, Paluch-Shimon S. et al. Ribociclib as first-line therapy for HR-positive, advanced breast cancer. N Engl J Med 2016; 375: 1738-48
- Goetz MP, Toi M, Campone M, Sohn J, Paluch-Shimon S, Huober J. et al. MONARCH 3: Abemaciclib as initial therapy for advanced breast cancer. J Clin Oncol 2017; 35: 3638-46
- Im SA, Lu YS, Bardia A, Harbeck N, Colleoni M, Franke F. et al. Overall survival with ribociclib plus endocrine therapy in breast cancer. N Engl J Med 2019; 381: 307-16
- Winters S, Martin C, Murphy D, Shokar NK. Breast cancer epidemiology, prevention, and screening. Prog Mol Biol Transl Sci 2017; 151: 1-32
- Rauthan A, Patil P, Somashekhar S, Zaveri S. Real-world single centre experience with palbociclib as first line treatment in Indian patients with metastatic breast cancer. J Clin Oncol 2018; 36: e13030
- Perez JE, Machiavelli M, Leone BA, Romero A, Rabinovich MG, Vallejo CT. et al. Bone-only versus visceral-only metastatic pattern in breast cancer: Analysis of 150 patients. A GOCS study. Am J Clin Oncol 1990; 13: 294-8
- Figura NB, Potluri TK, Mohammadi H, Oliver DE, Arrington JA, Robinson TJ. et al. CDK 4/6 inhibitors and stereotactic radiation in the management of hormone receptor positive breast cancer brain metastases. J Neurooncol 2019; 144: 583-9
- Zarbin M. Real Life outcomes vs. clinical trial results. J Ophthalmic Vis Res 2019; 14: 88-92
- Giuliano M, Schettini F, Rognoni C, Milani M, Jerusalem G, Bachelot T. et al. Endocrine treatment versus chemotherapy in postmenopausal women with hormone receptor-positive, HER2-negative, metastatic breast cancer: A systematic review and network meta-analysis. Lancet Oncol 2019; 20: 1360-9
- André F, Ciruelos E, Rubovszky G, Campone M, Loibl S, Rugo HS. et al. Alpelisib for PIK3CA-mutated, hormone receptor-positive advanced breast cancer. N Engl J Med 2019; 380: 1929-40
- Wander SA. et al. A multicenter analysis of abemaciclib after progression on palbociclib in patients (pts) with hormone receptor-positive (HR+)/HER2- metastatic breast cancer (MBC). J Clin Oncol 2019; 37: 1057
- Baselga J, Campone M, Piccart M, Burris 3rd HA, Rugo HS, Sahmoud T. et al. Everolimus in postmenopausal hormone-receptor-positive advanced breast cancer. N Engl J Med 2012; 366: 520-9
- Fang H, Huang D, Yang F, Guan X. Potential biomarkers of CDK4/6 inhibitors in hormone receptor-positive advanced breast cancer. Breast Cancer Res Treat 2018; 168: 287-97
- Del Re M, Bertolini I, Crucitta S, Fontanelli L, Rofi E, De Angelis C. et al. Overexpression of TK1 and CDK9 in plasma-derived exosomes is associated with clinical resistance to CDK4/6 inhibitors in metastatic breast cancer patients. Breast Cancer Res Treat 2019; 178: 57-62
- Finn RS, Liu Y, Zhu Z, Martin M, Rugo HS, Diéras V. et al. Biomarker analyses of response to cyclin-dependent kinase 4/6 inhibition and endocrine therapy in women with treatment-naïve metastatic breast cancer. Clin Cancer Res 2020; 26: 110-21
Address for correspondence
Publication History
Received: 22 July 2019
Accepted: 02 January 2020
Article published online:
23 May 2021
© 2020. Indian Society of Medical and Paediatric Oncology. This is an open access article published by Thieme under the terms of the Creative Commons Attribution-NonDerivative-NonCommercial-License, permitting copying and reproduction so long as the original work is given appropriate credit. Contents may not be used for commercial purposes, or adapted, remixed, transformed or built upon. (https://creativecommons.org/licenses/by-nc-nd/4.0/.)
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India

| Figure 1: Progression-free survival

| Figure 2: Overall survival
- 1 Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 Countries. CA Cancer J Clin 2018; 68: 394-424
- 2 Nair N, Shet T, Parmar V, Havaldar R, Gupta S, Budrukkar A. et al. Breast cancer in a tertiary cancer center in India – An audit, with outcome analysis. Indian J Cancer 2018; 55: 16-22
- 3 Matutino A, Joy AA, Brezden-Masley C, Chia S, Verma S. Hormone receptor-positive, HER2-negative metastatic breast cancer: Redrawing the lines. Curr Oncol 2018; 25: S131-41
- 4 Osborne CK, Schiff R. Mechanisms of endocrine resistance in breast cancer. Annu Rev Med 2011; 62: 233-47
- 5 AlFakeeh A, Brezden-Masley C. Overcoming endocrine resistance in hormone receptor-positive breast cancer. Curr Oncol 2018; 25: S18-27
- 6 Sedlacek H, Czech J, Naik R, Kaur G, Worland P, Losiewicz M. et al. Flavopiridol (L86 8275 NSC 649890), a new kinase inhibitor for tumor therapy. Int J Oncol 1996; 9: 1143-68
- 7 Tan AR, Yang X, Berman A, Zhai S, Sparreboom A, Parr AL. et al. Phase I trial of the cyclin-dependent kinase inhibitor flavopiridol in combination with docetaxel in patients with metastatic breast cancer. Clin Cancer Res 2004; 10: 5038-47
- 8 DiPippo AJ, Patel NK, Barnett CM. Cyclin-dependent kinase inhibitors for the treatment of breast cancer: Past, present, and future. Pharmacotherapy 2016; 36: 652-67
- 9 Finn RS, Martin M, Rugo HS, Jones S, Im SA, Gelmon K. et al. Palbociclib and letrozole in advanced breast cancer. N Engl J Med 2016; 375: 1925-36
- 10 Hortobagyi GN, Stemmer SM, Burris HA, Yap YS, Sonke GS, Paluch-Shimon S. et al. Ribociclib as first-line therapy for HR-positive, advanced breast cancer. N Engl J Med 2016; 375: 1738-48
- 11 Goetz MP, Toi M, Campone M, Sohn J, Paluch-Shimon S, Huober J. et al. MONARCH 3: Abemaciclib as initial therapy for advanced breast cancer. J Clin Oncol 2017; 35: 3638-46
- 12 Im SA, Lu YS, Bardia A, Harbeck N, Colleoni M, Franke F. et al. Overall survival with ribociclib plus endocrine therapy in breast cancer. N Engl J Med 2019; 381: 307-16
- 13 Winters S, Martin C, Murphy D, Shokar NK. Breast cancer epidemiology, prevention, and screening. Prog Mol Biol Transl Sci 2017; 151: 1-32
- 14 Rauthan A, Patil P, Somashekhar S, Zaveri S. Real-world single centre experience with palbociclib as first line treatment in Indian patients with metastatic breast cancer. J Clin Oncol 2018; 36: e13030
- 15 Perez JE, Machiavelli M, Leone BA, Romero A, Rabinovich MG, Vallejo CT. et al. Bone-only versus visceral-only metastatic pattern in breast cancer: Analysis of 150 patients. A GOCS study. Am J Clin Oncol 1990; 13: 294-8
- 16 Figura NB, Potluri TK, Mohammadi H, Oliver DE, Arrington JA, Robinson TJ. et al. CDK 4/6 inhibitors and stereotactic radiation in the management of hormone receptor positive breast cancer brain metastases. J Neurooncol 2019; 144: 583-9
- 17 Zarbin M. Real Life outcomes vs. clinical trial results. J Ophthalmic Vis Res 2019; 14: 88-92
- 18 Giuliano M, Schettini F, Rognoni C, Milani M, Jerusalem G, Bachelot T. et al. Endocrine treatment versus chemotherapy in postmenopausal women with hormone receptor-positive, HER2-negative, metastatic breast cancer: A systematic review and network meta-analysis. Lancet Oncol 2019; 20: 1360-9
- 19 André F, Ciruelos E, Rubovszky G, Campone M, Loibl S, Rugo HS. et al. Alpelisib for PIK3CA-mutated, hormone receptor-positive advanced breast cancer. N Engl J Med 2019; 380: 1929-40
- 20 Wander SA. et al. A multicenter analysis of abemaciclib after progression on palbociclib in patients (pts) with hormone receptor-positive (HR+)/HER2- metastatic breast cancer (MBC). J Clin Oncol 2019; 37: 1057
- 21 Baselga J, Campone M, Piccart M, Burris 3rd HA, Rugo HS, Sahmoud T. et al. Everolimus in postmenopausal hormone-receptor-positive advanced breast cancer. N Engl J Med 2012; 366: 520-9
- 22 Fang H, Huang D, Yang F, Guan X. Potential biomarkers of CDK4/6 inhibitors in hormone receptor-positive advanced breast cancer. Breast Cancer Res Treat 2018; 168: 287-97
- 23 Del Re M, Bertolini I, Crucitta S, Fontanelli L, Rofi E, De Angelis C. et al. Overexpression of TK1 and CDK9 in plasma-derived exosomes is associated with clinical resistance to CDK4/6 inhibitors in metastatic breast cancer patients. Breast Cancer Res Treat 2019; 178: 57-62
- 24 Finn RS, Liu Y, Zhu Z, Martin M, Rugo HS, Diéras V. et al. Biomarker analyses of response to cyclin-dependent kinase 4/6 inhibition and endocrine therapy in women with treatment-naïve metastatic breast cancer. Clin Cancer Res 2020; 26: 110-21


 PDF
PDF  Views
Views  Share
Share

