Malignant lymphoma in Eastern India: A retrospective analysis of 455 cases according to World Health Organization classification
CC BY-NC-ND 4.0 · Indian J Med Paediatr Oncol 2013; 34(04): 242-246
DOI: DOI: 10.4103/0971-5851.125235
Abstract
Background: Malignant lymphoma (ML) is one of the most common cancers and is most prevalent in developed countries. The distribution of different subtypes of ML varies in the different geographical locations according to World Health Organization (WHO) Classification. Aims and Objectives : The study was aimed to analyze the different patterns of ML in Eastern India and to compare it with other geographical locations. Materials and Methods: Four hundred and fifty five patients of two large hospitals in Eastern India were included over a period of four years and were categorized according to WHO classification, using the morphology and immunohistochemistry. Results: There were 347 (76.3%) non Hodgkin lymphomas (NHL), and 108 (23.7%) Hodgkin lymphomas (HL). Diffuse large B-cell lymphoma was the most common of the NHL type (35.2%) followed by the follicular lymphoma (19.3%). B-cell lymphoblastic lymphoma was the least common type of NHL (1.4%). Mixed cellularity (33.3%) and nodular sclerosis (26.9%) were the two most common type of HL. Childhood lymphoma comprised of 12.5%of all ML. T-cell NHL and HL were the common lymphomas in this age group. Conclusion: Incidence of follicular lymphoma is lower compared to western studies and mixed cellularity is the most common subtype of HL unlike nodular sclerosis subtype in Western world. Burkitt′s type NHL though is the most common subtype of childhood ML in many studies. However, in our study, T-cell NHL is the most common type of childhood ML.
Publication History
Article published online:
19 July 2021
© 2013. Indian Society of Medical and Paediatric Oncology. This is an open access article published by Thieme under the terms of the Creative Commons Attribution-NonDerivative-NonCommercial-License, permitting copying and reproduction so long as the original work is given appropriate credit. Contents may not be used for commercial purposes, or adapted, remixed, transformed or built upon. (https://creativecommons.org/licenses/by-nc-nd/4.0/.)
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India
Abstract
Background:
Malignant lymphoma (ML) is one of the most common cancers and is most prevalent in developed countries. The distribution of different subtypes of ML varies in the different geographical locations according to World Health Organization (WHO) Classification.
Aims and Objectives:
The study was aimed to analyze the different patterns of ML in Eastern India and to compare it with other geographical locations.
Materials and Methods:
Four hundred and fifty five patients of two large hospitals in Eastern India were included over a period of four years and were categorized according to WHO classification, using the morphology and immunohistochemistry.
Results:
There were 347 (76.3%) non Hodgkin lymphomas (NHL), and 108 (23.7%) Hodgkin lymphomas (HL). Diffuse large B-cell lymphoma was the most common of the NHL type (35.2%) followed by the follicular lymphoma (19.3%). B-cell lymphoblastic lymphoma was the least common type of NHL (1.4%). Mixed cellularity (33.3%) and nodular sclerosis (26.9%) were the two most common type of HL. Childhood lymphoma comprised of 12.5%of all ML. T-cell NHL and HL were the common lymphomas in this age group.
Conclusion:
Incidence of follicular lymphoma is lower compared to western studies and mixed cellularity is the most common subtype of HL unlike nodular sclerosis subtype in Western world. Burkitt's type NHL though is the most common subtype of childhood ML in many studies. However, in our study, T-cell NHL is the most common type of childhood ML.
INTRODUCTION
The World Health Organization (WHO) classification of malignant lymphoma (ML) has become popular since its introduction in 2001 and has been applied to the classification of ML in different countries around the world.[1] Different studies from America, Europe, Jordan, Iran, Japan, China, India, Iraq have revealed that the relative proportion of various ML according to WHO classification differ with geographical regions.[2,3,4,5]
Only a few studies of ML are undertaken in India so far.[6,7] To the best of our knowledge, this is the first study from Eastern India. The present study was undertaken to subtype the ML in this part of the world according to WHO classification and to compare it with other studies to find out geographical variation of ML.[8,9]
MATERIALS AND METHODS
In the present study, 455 cases of malignant lymphomas, which included various subtypes of non-Hodgkin lymphoma (NHL) and Hodgkin lymphoma (HL); collected from two large hospitals over a period of four years (2008 to 2011). Of these two hospitals, one is a Tertiary Care Cancer Hospital of Eastern India and another is the medical college and hospital. Additional clinical data including, age, gender, and site of sampling were recorded.
All cases were reviewed by three pathologists, which included the first author. Tissue sections of 3-4 μ thickness from paraffin embedded tissue and decalcified bone marrow specimens (trephine biopsy) were examined by hematoxylin and eosin and a panel of immunocytological marker using the streptavidin-biotin peroxidises method. The panel of immunohistochemistry (IHC) markers included CD45 (LCA), CD45RO, CD79, CD20, CD23, CD10, CD5, CD3, CD56, CD4, CD8, CD15, CD30, CD34, EMA, MUM1, BCL6, TdT, BCL2, MIB1/KI67, ALK1, Kappa and lambda light chains.[10,11] Other IHC markers were also utilized if needed for evaluation of some unknown neoplasm/ML.
All patients were subjected to routine hematological (estimation of hemoglobin, total, and different leucocyte count, platelet count, peripheral smear for abnormal/blast cells etc.) and biochemical (liver function tests, urea, creatinine, uric acid) investigations. The radiological examination included chest radiograph, computed tomography, and ultrasonography of abdomen. Reticulin stain was carried out in trephine biopsies to assess the marrow involvement by neoplastic cells and reactive fibrosis.
RESULTS
There are 455 cases of ML in the present study. NHL was diagnosed in 347 cases (76.3%) and HL in 108 cases (23.7%). Out of these 455 cases of ML adult cases were 398 (NHL 216 and HL 92) and pediatric cases (< 15 years) were 57 (NHL 41 and HL 16). Male-female ratio was 345:110 = 3.1:1 and the NHL: HL ratio was 3.2:1.
NHL
Among 347 cases of NHL 218 patients were males (62.8%) and 69 were females (19.9%) and male to female ratio was 3.2:1. The age range was 2-79 years with a mean age of 39.4 years. Among NHL cases B-cell lymphomas were the predominant type (257 cases) accounting for 74.1% of all cases [Table 1]. T-cell lymphomas constitute 90 cases (25.9%). Of the B-cell neoplasms, diffuse large B-cell lymphoma (DLBCL) was the most common subtype (122, 35.2%) cases, followed by follicular lymphoma (67 cases, 19.3%). Among the follicular lymphomas, Grade 3 was most common type (28 cases), followed by Grade 2 (25 cases) and Grade 1 (14 cases). Third common B-cell NHL was Burkitt's lymphoma (20 cases, 5.8%) followed by small lymphocytic lymphoma (19 cases, 5.5%).
Table 1
Distribution of types of 347 patients with NHL according to the WHO classification
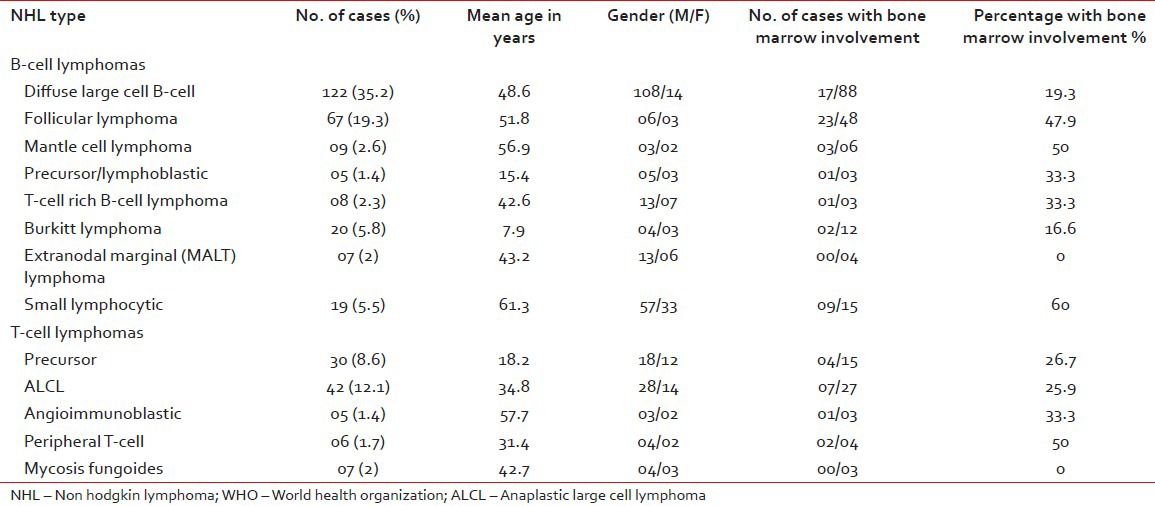
T-cell NHL comprised of 90 cases (25.9%) and anaplastic large cell lymphoma (ALCL) was the most common subtype (42 cases, 12.1%), followed by precursor T lymphoblastic lymphoma (30 cases, 8.6%). Other T-cell NHL were Mycosis Fungoides (7 cases, 2%), peripheral T-cell lymphomas (6 cases, 1.7%) and angioimmunoblastic (5 cases, 1.4%).
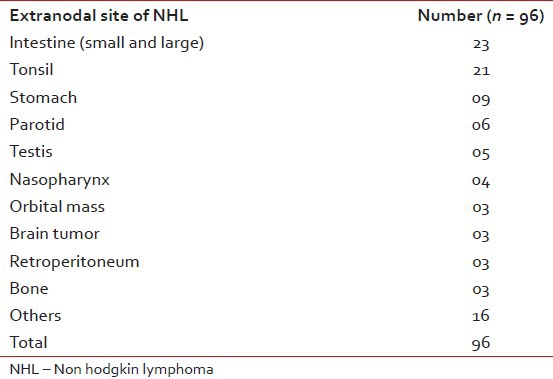
A total of 251 cases (72.3%) were nodal and 96 cases (27.7%) were extranodal. Cervical lymph node was the most common site (52 cases, 20.7%) followed by axillary nodes (36 cases, 14.35), the inguinal nodes (20 cases, 7.9%), the mediastinal nodes (14 cases, 5.6%), abdominal nodes (13 cases, 5.2%) and others. The most frequent extra nodal site was intestine (23 cases, 23.9%), followed by tonsil (21 cases, 21.9%), stomach (9 cases, 9.4%). parotid (6 cases, 6.2%) and others.
HL
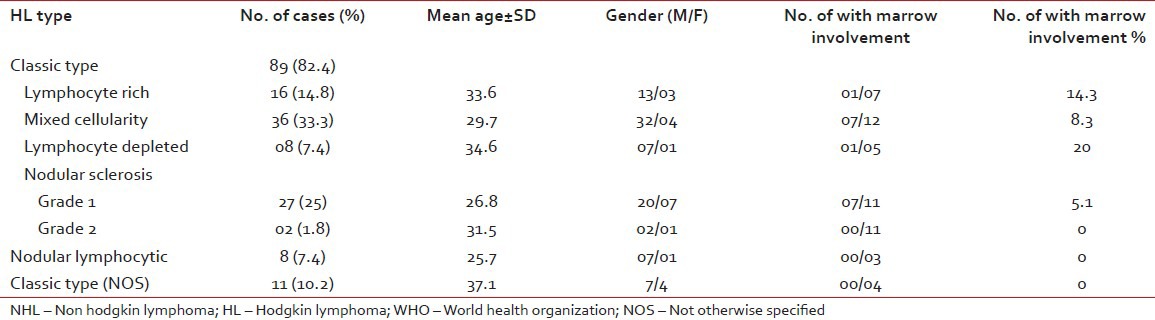
Out of 108 cases of HL, 88 patients were males and 20 patients were females with a male-female ratio of 4.4:1. The age range for HL was 3-67 years with a mean age range of 31.3 years. All cases of HL were of nodal origin and no extra nodal case was detected in the present study. The most frequent site was cervical group of lymph nodes (71 cases, 65.7%) followed by axillary nodes (23 cases, 21.3%), mediastinal nodes (9 cases, 8.3%) and others.
Mixed cellularity variant was the commonest type (36cases, 33.3%). followed by nodular sclerosis (29 cases, 26.8%), lymphocytic rich (16 cases, 14.8%), classic not otherwise specified (NOS) (11 cases, 10.2%) and lymphocyte depleted (8 cases, 7.4%) [Table 2].
Table 2
Extranodal NHL distribution according to the primary site
Among the 29 cases of nodular sclerosis 27 cases were Grade 1 and two cases were Grade 2. There were 11 cases of classic HL, which could not be subtyped due to small tissue specimen, and they were classified into classic NOS type.
Bone marrow involvement
Out of the 347 cases of NHL, trephine biopsy material was available in 231 cases and marrow involvement was noted in 70 cases (30.3%). Highest marrow involvement was seen in small lymphocytic lymphoma type NHL (60%) followed by mantle cell lymphoma (50%), follicular lymphoma (47.9%) [Table 1].
Among the HL trepine biopsies were available in 43 cases out of total 108 cases and marrow involvement was detected in 4 cases (9.3%) [Table 2].
Extranodal NHL
Out of 347 cases of NHL; extra nodal cases were found in 96 cases (27.7%). The most frequent affected site was the small and large intestine (23 cases, 23.9%) followed by the tonsil (21 cases,21.8%) other sites were stomach (9 cases, 9.4%), parotid (6 cases, 6.4%), testis (5 cases, 5.2%), nasopharynx (4 cases, 4.2%), orbit (3 cases, 3.1%), brain (3 cases, 3.1%), etc. [Table 3].
Table 3
Distribution of types of 347 patients with NHL according to the WHO classification
Childhood lymphoma
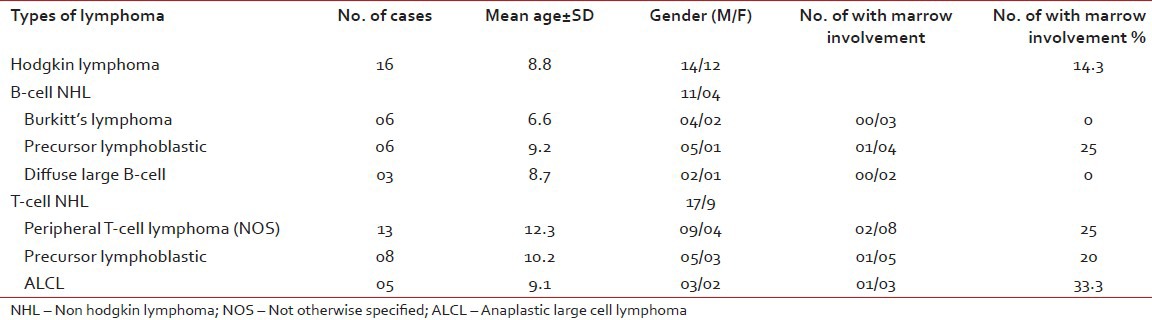
In the present study, childhood lymphoma ( < 15 years of age) comprised of 57 cases (1.25%) cases out of total 455 cases. In this age group commonest type of ML was HL (16 cases, 28%) followed by the peripheral T-cell lymphoma (13 cases, 22.8%), precursor lymphoblastic T-cell lymphoma (8 cases, 14%), Burkitt's lymphoma (6 cases, 10.5%), precursor lymphoblastic B-cell lymphoma (6 cases, 10.5%) and others [Table 4]. There were 42 male patients and 15 female patients with a male female ratio of 2.8:1. The age range was 7 months - 14 years and median age was 9.3 years. Trephine biopsy examination was carried out in 32 cases, out of which 6 cases showed bone marrow involvement (18.7%). Highest marrow involvement was seen in ALCL (33.3%) followed by precursor lymphoblastic B-cell lymphoma (25%) and peripheral T-cell lymphoma (25%) [Table 4].
Table 4
Distribution of 57 cases of various childhood lymphomas
DISCUSSION
The classification of malignant lymphomas has changed over the years and WHO classification or WHO classification with minor modifications has been adopted in most centers.[1] By using the WHO classification with IHC evaluation, both NHL and HL are easily classified.[12] Classifying ML according to B and T-cell type has prognostic significance to the oncologists.[13] Due to the limited availability of the panel of IHC markers, WHO classification of ML is being studied in very few centers of Eastern India. The importance of the current study is that it documents the various types of ML based on the WHO classification in a geographical area (Eastern India) that has not been previously investigated.
In India, the first population based cancer registry was established in Mumbai (Bombay) by the Indian Cancer Society in 1964. Other urban registries available are Delhi, Chennai, Bhopal and Bangalore under the network of National Cancer Registry Program (NCRP) of Indian Council of Medical Research.[14] However, no such urban cancer registry is available from Eastern India.
In our study, HL comprised 23.7% of ML cases, and ratio of NHL to HL is 3.2:1. In another Indian study, this ratio was 1.58:1 and reported a higher percentage of HL (38.7%) compared to our study (23.7%).[6] The rate of HL is lower than west (30%)[1] and almost similar in Jordan (21.6%) and Northern Iran (24%).[2,15] A higher percentage (41%) of HL was found in UAE.[15] Much lower frequency of HL was seen in some Asian countries like Japan (7%), Thailand (8.5%), and China (6.6%).[16,17] In our study, mixed cellularity is the most common subtype (33.3%) followed by the modular sclerosis subtype (26.8%). Similar trend was found in Pakistan and also in one Indian study.[6,18] However, in Jordan, Europe, USA and other western world, modular sclerosis is the most common subtype.[1,15] Getachew A reported lymphocyte predominant subtype as the common type of HL in Western Ethiopia of Africa.[19] Increased incidence of mixed cellularity subtype in India, Pakistan and other Asian countries may be due higher risk of childhood exposure to Epstein Barr virus that is more likely to be associated with a mixed cellularity than nodular sclerosis.[20] With regards to specific subtypes of NHL, the present study showed DLBCL is the most common subtype (35.2%) which is comparable to USA and Europe (25-30%).[1,21] The trend is similar in Jordan (28.2%), China (3.5:1) in India.[11,22] However, a higher proportion of DLBCL was noted in northern Iraq (52.2%), UAE (59%) and in Pakistan (66.1%).[2,11,18]
FL was the second most common subtype of NHL (19.3%) in our study, which is comparable in other Indian study.[22] A higher proportion is noted in Western studies (28-32%). Lower incidence of FL (4-8%) was reported from Saudi Arabia, Egypt, UAE, North Jordan and Pakistan.[2,18] Naresh et al. opined that the low rates of FL in developing countries might be due to many DLBCL progressed from previously undiagnosed FL and that unique environmental or genetic factors may have contributed to such progression.[22]
Bone Marrow involvement was noted in 30.3% of NHL cases and 9.3% of HL cases. Bone marrow involvement in NHL varies from 20% to 40% in different Western studies, which is similar to our studies.[1,6,23] In, different Indian studies bone marrow involvement in HL is 8.33-9.68% which is almost similar to our study.[6,7] Extranodal lymphomas (ENL) are common and accounted for 27.7% of ML cases. This figure is close to the incidence in USA (26%) and slightly lower than Jordan (30.5%), UAE (29%) and Bahrain (41%).[11] DLBCL was the most common subtype of ENL and gastrointestinal tract is the most common site in a study in Kuwait, similar to our findings.[24] However, higher incidence of ENL (44.9%) was seen in Taiwan.[25] While NHL and HL have long been regarded as distinct entities, recent study showed a closer association of these two entities. The analysis of cases in which both diseases are present in the same anatomic site (composite lymphomas), or in separate sites (sequential or simultaneous NHL and HL), indicates that this phenomenon occurs more frequently than would be expected by chance alone.[26,27]
CONCLUSION
The present study from Eastern Indian shows higher number of NHL cases (76.3%) then HL cases (23.7%) and male–female ratio is 3.1:1. A lower percentage of follicular lymphoma is seen compared to Western studies and Grade 3 is the most common subtype of follicular lymphoma. Among the extranodal NHL, tonsil is most frequently involved site and is second most common site of extranodal NHL in our study. Mixed cellularity type of HL is the most common subtype like in other Asian, and Indian studies; however, unlike western studies where nodular sclerosis is the commonest type. T-cell NHL is the most common type of childhood NHL unlike Burkitt's lymphoma in other studies. Highest bone marrow involvement is seen in mantle cell lymphoma among NHLs and lymphocyte depleted variant among HLs and ALCL among childhood lymphomas.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.


 PDF
PDF  Views
Views  Share
Share

