Metastatic Merkel Cell Carcinoma of the Abdominal Wall
CC BY-NC-ND 4.0 · Indian J Med Paediatr Oncol 2020; 41(02): 275-279
DOI: DOI: 10.4103/ijmpo.ijmpo_165_18
Abstract
Merkel-cell carcinoma (MCC) is a rare skin malignancy seen in elderly males. It is a highly aggressive tumor with a poor prognosis. Surgery is the mainstay of treatment for localized disease with adjuvant radiation depending on the locoregional extent, while chemotherapy has a role in metastatic disease. Emerging data from treatment with immune checkpoint inhibitors look promising. We report a case of MCC in an elderly male diagnosed and treated with chemotherapy and radiation, with a review of the literature of this rare malignancy.
Keywords
Merkel cell carcinoma - neuroendocrine tumor - skin cancerPublication History
Received: 24 July 2018
Accepted: 14 October 2019
Article published online:
23 May 2021
© 2020. Indian Society of Medical and Paediatric Oncology. This is an open access article published by Thieme under the terms of the Creative Commons Attribution-NonDerivative-NonCommercial-License, permitting copying and reproduction so long as the original work is given appropriate credit. Contents may not be used for commercial purposes, or adapted, remixed, transformed or built upon. (https://creativecommons.org/licenses/by-nc-nd/4.0/.)
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India
Abstract
Merkel-cell carcinoma (MCC) is a rare skin malignancy seen in elderly males. It is a highly aggressive tumor with a poor prognosis. Surgery is the mainstay of treatment for localized disease with adjuvant radiation depending on the locoregional extent, while chemotherapy has a role in metastatic disease. Emerging data from treatment with immune checkpoint inhibitors look promising. We report a case of MCC in an elderly male diagnosed and treated with chemotherapy and radiation, with a review of the literature of this rare malignancy.
Keywords
Merkel cell carcinoma - neuroendocrine tumor - skin cancerIntroduction
Merkel cell carcinoma (MCC) is a primary cutaneous neuroendocrine carcinoma, commonly seen in elderly males. It is highly aggressive, rare malignant tumor of the skin with threefold increase in incidence as per the Western literature.[1] It is the second leading cause of nonmelanoma skin cancer death with 30% mortality.[2]
The common features for diagnosing MCC go with acronym: AEIOU (asymptomatic/painless, rapidly expanding (<3 months), immunosuppression, older than 50, and location on an ultraviolet (UV)-exposed site), wherein three or more criteria are seen in 89% of cases.[3] Regional nodal involvement has high propensity for local recurrence and distant metastases involving the skin, liver, lung, bone, and brain in one-third patients. The treatment of MCC includes surgery, radiotherapy, and chemotherapy/immunotherapy depending on the presence or absence of metastases.
We report a case of metastatic MCC of the abdominal wall in a 56-year-old male.
Case Report
A 56-year-old gentleman presented with painless progressive swelling on the left side of the abdomen for 6 months, associated with skin ulceration and discharge without any constitutional symptoms. Contrast-enhanced computed tomography (CECT) of the abdomen showed 166 mm × 123 mm × 45 mm heterogeneously enhancing anterior abdominal mass in the left lumbar region, with loss of fat plane between the mass and underlying muscle. The lesion was extending up to the skin with focal areas of ulceration, without intraperitoneal or retroperitoneal extension. CECT of the chest revealed well-defined heterogeneously enhancing lesions in the left lower lobe suggestive of metastases. Biopsy from the primary lesion showed medium-sized neoplastic cells arranged in sheets [Figure 1] with scanty cytoplasm [Figure 2] and round-to-oval nuclei with inconspicuous nucleoli consistent with poorly differentiated malignancy. Immunohistochemistry (IHC) showed leukocyte common antigen-negative, Melan A-negative, pancytokeratin dot-like positive [Figure 3], CD34-negative, cytokeratin 20 (CK20) dot-like positivity [Figure 4], CD 56-positive, CD 117-negative, and chromogranin-negative. Morphology with IHC correlation was suggestive of MCC. Brain and bone scans were normal.
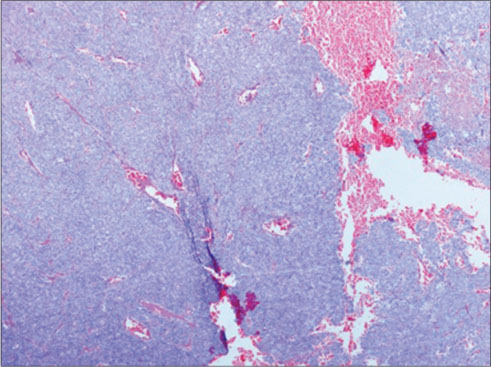
| Figure 1: Cellular lesion arranged predominantly in sheets, (H and E, ×40)
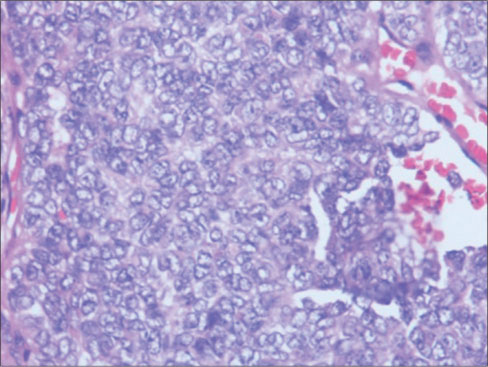
| Figure 2: Monotonous population of cells having scanty cytoplasm, round vesicular nucleus with finelygranular chromatin with marginated nucleoli, (H and E × 400)
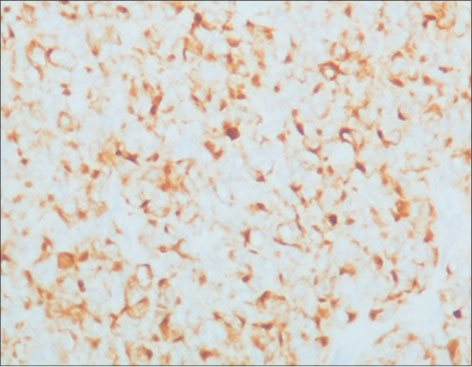
| Figure 3: Pancytokeratin immunostaining showing cytoplasmic positivity and focal dot like positivity
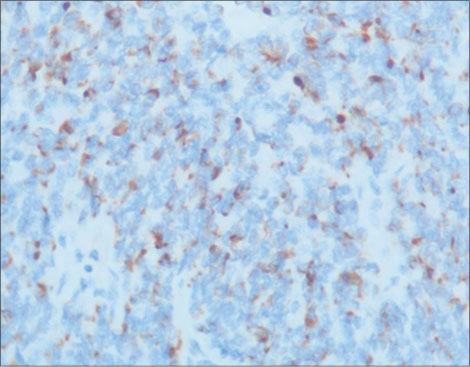
| Figure 4: CK20 immunostaining shows characteristic perinuclear dot-like positivity
Two cycles of cisplatin/etoposide-based chemotherapy showed a marginal response hence planned to integrate radiation. Concurrent chemoradiation up to 55 Gy in 20 fractions with weekly cisplatin was administered. A good clinical response with 75% tumor regression and improvement in performance status was observed at the end of 10 weeks [Picture 1] and [Picture 2]. Systemic chemotherapy was continued after radiation.
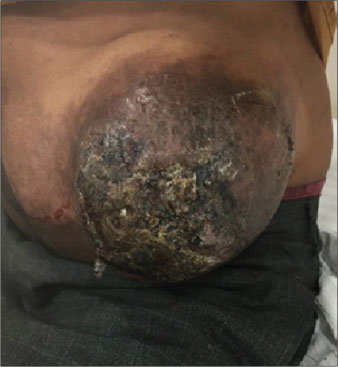
| Picture 1: Clinical image of the swelling at diagnosis
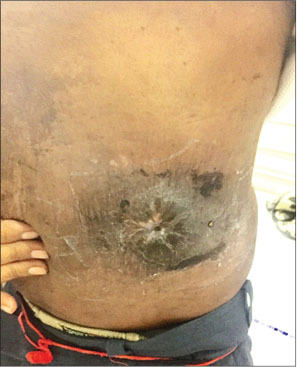
| Picture 2: Clinical image of the swelling after treatment
Discussion
MCC is a rare aggressive tumor of the dermis first reported by Toker in 1972 as trabecular carcinoma of the skin. Tang and Toker (1978) found dense core granules in the cytoplasm of the tumor cells by electron microscopy which led to the hypothesis that this tumor arises from Merkel cells.[4] The name MCC was coined by De Wolff-Peeters et al. in 1980.[5]
Danish registry reports a 5.4 times increase in the incidence of MCC over 18 years.[6]
MCC is commonly seen in elderly Caucasians with a mean age of 70 years without sex predilection. The skin of the head and neck is affected in 50% cases, extremities in 40%, and trunk and mucosa in 10%.[7] Risk factors include sunlight exposure and immunosupression. Merkel cell polyomavirus (MCPyV) sequences were detected in 80% of MCC tumors when compared 16% in control skin tissues and suggesting the possibility of viral infection as a contributing factor in pathogenesis that could have triggered clonal expansion of the tumor cells.[8]
Although the initial infection may occur in childhood, virus-positive MCC typically does not occur until age 70 years. Virus-positive MCC has a specific integration pattern and it expresses a large T-cell antigen in tumor cells which inactivates p53 and Rb. They have extremely low mutational burdens, in contrast to UV-induced MCPyV-negative MCCs, which are characterized by a mutational load that is 100 times higher. Viral antigens are foreign and thus potentially strong immune stimulants, and many virus-associated tumors are characterized by robust immune infiltrates and PD-L1 expression.[9]
MCC typically develops as a painless, nontender rapidly growing nodule or plaque, seen on sun-exposed areas of the body. Nodules are solitary, firm, fleshy to reddish-blue, having a smooth shiny surface.
Histologically, the tumor is composed of strands or nests of monotonously uniform round blue cells containing large basophilic nuclei, inconspicuous nucleoli, and minimal cytoplasm. Intermediate type, small cell type, and trabecular type are the three main histological patterns.
IHC of MCC demonstrates epithelial and neuroendocrine markers. The loosely arranged intermediate filaments stain for CKs. Paranuclear dot-like pattern of CK20 expression due to clumping of intermediate filaments is highly specific of MCC. MCC also stains neuroendocrine markers CD56, chromogranin, neuron-specific enolase, and synaptophysin. S100 and other melanoma markers are negative. Thyroid transcription factor-1 differentiates it from metastatic small cell carcinoma of the lung. Strong diffuse positivity of p63 has negative prognostic implication.[10]
Squamous or sarcomatoid differentiation may be seen occasionally.
The staging of MCC according to American Joint Committee on Cancer (AJCC) eighth edition is shown in [Table 1].[11] Radiological classification based on local and distant metastasis has been proposed (Stage 1: cutaneous involvement, Stage 2: regional nodal invasion, and Stage 3: systemic metastases).[12] Sentinel node mapping for the regional extent and positron-emission tomography (PET) computed tomography scan for the evaluation of distant metastasis is recommended. The rationale for the implementation of somatostatin receptor scintigraphy (SRS) in patients with MCC is based on the neuroendocrine characteristics of the malignancy.
Table 1Merkel-cell carcinoma staging system (American Joint Committee on Cancer, eighth edition)
|
Stage |
TNM |
OS 2 years (%) |
OS 5 years (%) |
|---|---|---|---|
|
OS – Overall survival; TNM – Tumor, node, metastasis |
|||
|
Stage I |
Primary <2 cm (T1) |
67 |
81 |
|
Stage II |
Primary 2 cm or more (T2) |
59 |
67 |
|
Stage III |
Nodal disease (N1) |
49 |
52 |
|
Stage IV |
Systemic metastases (M1) |
23 |
11 |
Poor prognostic factors include male sex, size of the primary tumor >2 cm, and metastatic disease.
The treatment of MCC includes surgery, radiotherapy, and chemotherapy depending on the presence or absence of metastatic disease. For localized disease, wide local excision and sentinel node dissection are the standard of care. Mohs micrographic surgery has been recommended for localized lesions with excellent cosmetic results.
Adjuvant radiotherapy (40–60 Gy) to the primary site and regional nodes reduces the risk of local recurrence and increases median survival.[14] Adjuvant chemoradiotherapy has been reported to result in improved overall survival when compared to adjuvant radiotherapy in patients with positive margins, tumor size at least 3 cm, and male sex.[15] Palliative radiation therapy (RT) can be considered for inoperable tumors.
Chemotherapy is recommended for inoperable and metastatic disease. In view of the neuroendocrine features, chemotherapy with platinum/etoposide shows better response. Chemotherapeutic agents such as cyclophosphamide, doxorubicin, vincristine, methotrexate, and 5-fluorouracil have been tried. In Trans-Tasman Radiation Oncology Group study, concurrent carboplatin/etoposide with radiation showed good locoregional control and survival.[16] However, in general, there is an initial regression followed by recurrence within 4–15 months.
Biologic agents such as interferons, tumor necrosis factor, and hyperthermia were used in advanced disease. Coexpression of c-KIT in a high percentage of MCC led to theuse of tyrosine kinase inhibitors such as imatinib with promising results.[17] [18] Somatostatin analogs showed objective responses with moderate doses and minimal side effects with survivals over 10 months.[19]
Peptide receptor radionuclide therapy as a new tool in the management of inoperable or metastatic patients with 111 indium-, 90 yttrium- or 177 lutetium-labeled somatostatin analogs has been highlighted in several case reports. 90Y-DOTATOC and 177 Lu-DOTATATE are the most promising, providing long-lasting responses and good survival rates.[20]
There are several ongoing clinical trials of immune checkpoint inhibitors (anti-PD-1, anti-PD-L1, and CTLA-4 abs) administered as monotherapy or in combination in metastatic and adjuvant[21] settings. Studies have shown that around 50% of MCCs express PD-1 on tumor-infiltrating lymphocytes and PDL1 on tumor cells. Pembrolizumab therapy in advanced MCC is associated with a 56% objective response rate, including a 16% complete response (CR) rate; virus-associated tumors had an overall response rate (ORR) of 62% compared to 44% with virus-negative tumors.[22] Similarly, avelumab (PDL1) has shown ORR of 62%, 2 years’ progression-free survival (PFS) of 26%, and OS of 36% in a phase 2 trial.[23] In patients who had received at least one line of chemotherapy, avelumab (anti-PDL1 antibody) has shown an ORR of 33%, with a CR rate of 11% and has been Food and Drug Administration approved. However, it is too early to determine the long-term outcomes of these patients, and there are subsets of patients either refractory to immune checkpoint inhibitors or develop acquired resistance over time. Finally, the combination of conventional cytotoxic chemotherapy and RT in conjunction with immunotherapy remains to be determined.
Our case with a rare site of the presentation was a diagnostic and a therapeutic challenge.
Learning points
MCC is a primary neuroendocrine tumor that arises in the skin. It may occur at non sun-exposed sites as well
CK20 expression and paranuclear dot-like pattern of intermediate filament staining are highly suggestive of MCC
Surgery is the mainstay of treatment for localized disease, and it is usually followed by adjuvant radiation
Our case demonstrated a good clinical response, and PFS (more than 10 months and which is ongoing at the time of writing) can be achieved with cytotoxic chemotherapy and radiation
Immune checkpoint inhibitors offer new hope and should be used for durable clinical response.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form, the patient has given his consent for his images and other clinical information to be reported in the journal. The patient understands that name and initials will not be published and due efforts will be made to conceal identity, but anonymity cannot be guaranteed.
Conflict of Interest
There are no conflicts of interest.
Acknowledgment
The authors would like to thank the Department of medical oncology, BIACHRC, Hyderabad.
References
- Schrama D, Ugurel S, Becker JC. Merkel cell carcinoma: Recent insights and new treatment options. Curr Opin Oncol 2012; 24: 141-9
- Miller RW, Rabkin CS. Merkel cell carcinoma and melanoma: Etiological similarities and differences. Cancer Epidemiol Biomarkers Prev 1999; 8: 153-8
- Heath M, Jaimes N, Lemos B, Mostaghimi A, Wang LC, Peñas PF. et al. Clinical characteristics of Merkel cell carcinoma at diagnosis in 195 patients: The AEIOU features. J Am Acad Dermatol 2008; 58: 375-81
- Toker C. Trabecular carcinoma of the skin. Arch Dermatol 1972; 105: 107-10
- De Wolff-Peeters C, Marien K, Mebis J, Desmet V. A cutaneous APUDoma or Merkel cell tumor? A morphologically recognizable tumor with a biological and histological malignant aspect in contrast with its clinical behavior. Cancer 1980; 46: 1810-6
- Lyhne D, Lock-Andersen J, Dahlstrøm K, Drzewiecki KT, Balslev E, Muhic A. et al. Rising incidence of Merkel cell carcinoma. J Plast Surg Hand Surg 2011; 45: 274-80
- Cirillo F, Vismarra M, Cafaro I, Martinotti M. Merkel cell carcinoma: A retrospective study on 48 cases and review of literature. J Oncol 2012; 2012: 749030
- Feng H, Shuda M, Chang Y, Moore PS. Clonal integration of a polyomavirus in human merkel cell carcinoma. Science 2008; 319: 1096-100
- DeCaprio JA. Merkel cell polyomavirus and Merkel cell carcinoma. Philos Trans R Soc Lond B Biol Sci 2017; 372: pii-20160276
- Wong HH, Wang J. Merkel cell carcinoma. Arch Pathol Lab Med 2010; 134: 1711-6
- Amin MB, Greene FL, Edge SB, Compton CC, Gershenwald JE, Brookland RK. et al. The eighth edition AJCC Cancer Staging Manual: continuing to build a bridge from a population-based to a more “personalized” approach to cancer staging. CA Cancer J Clin 2017; 67: 93-9
- Yiengpruksawan A, Coit DG, Thaler HT, Urmacher C, Knapper WK. Merkel cell carcinoma. Prognosis and management. Arch Surg 1991; 126: 1514-9
- Kritikos N, Priftakis D, Stavrinides S, Kleanthous S, Sarafianou E. Nuclear medicine techniques in merkel cell carcinoma: A case report and review of the literature. Oncol Lett 2015; 10: 1610-6
- Strom T, Carr M, Zager JS, Naghavi A, Smith FO, Cruse CW. et al. Radiation therapy is associated with improved outcomes in Merkel cell carcinoma. Ann Surg Oncol 2016; 23: 3572-8
- Chen MM, Roman SA, Sosa JA, Judson BL. The role of adjuvant therapy in the management of head and neck Merkel cell carcinoma: An analysis of 4815 patients. JAMA Otolaryngol Head Neck Surg 2015; 141: 137-41
- Poulsen M, Rischin D, Walpole E, Harvey J, Mackintosh J, Ainslie J. et al. High-risk merkel cell carcinoma of the skin treated with synchronous carboplatin/etoposide and radiation: A Trans-Tasman Radiation Oncology group study – TROG 96:07. J Clin Oncol 2003; 21: 4371-6
- Ito Y, Kawamura K, Miura T, Ueda K, Onodera H, Takahashi H. et al. Merkel cell carcinoma. A successful treatment with tumor necrosis factor. Arch Dermatol 1989; 125: 1093-5
- Su LD, Fullen DR, Lowe L, Uherova P, Schnitzer B, Valdez R. et al. CD117 (KIT receptor) expression in merkel cell carcinoma. Am J Dermatopathol 2002; 24: 289-93
- di Bartolomeo M, Bajetta E, Buzzoni R, Mariani L, Carnaghi C, Somma L. et al. Clinical efficacy of octreotide in the treatment of metastatic neuroendocrine tumors. A study by the Italian trials in medical oncology group. Cancer 1996; 77: 402-8
- National Comprehensive Cancer Network. Clinical Practice Guideline Sinoncology. Available from: http://www.nccn.org/professionals/physician_gls/f_guidelines.asp. [Last accessed on 2019 July 25]
- NCCN Clinical Practice Guidelines in Oncology. Merkel Cell Carcinoma. Ver. 1. 2019th ed. Fort Washington, PA: National Comprehensive Cancer Network, Inc.; 2019. Available from: https://www.nccn.org/professionals/physician_gls/pdf/mcc.pdf. [Last accessed on 2019 Oct 29].
- Nghiem PT, Bhatia S, Lipson EJ, Kudchadkar RR, Miller NJ, Annamalai L. et al. PD-1 blockade with pembrolizumab in advanced Merkel-cell carcinoma. N Engl J Med 2016; 374: 2542-52
- Kaufman HL, Russell JS, Hamid O, Bhatia S, Terheyden P, D’Angelo SP. et al. Updated efficacy of avelumab in patients with previously treated metastatic Merkel cell carcinoma after≥1 year of follow-up: JAVELIN Merkel 200, a phase 2 clinical trial. J Immunother Cancer 2018; 6: 7
Address for correspondence
Publication History
Received: 24 July 2018
Accepted: 14 October 2019
Article published online:
23 May 2021
© 2020. Indian Society of Medical and Paediatric Oncology. This is an open access article published by Thieme under the terms of the Creative Commons Attribution-NonDerivative-NonCommercial-License, permitting copying and reproduction so long as the original work is given appropriate credit. Contents may not be used for commercial purposes, or adapted, remixed, transformed or built upon. (https://creativecommons.org/licenses/by-nc-nd/4.0/.)
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India

| Figure 1: Cellular lesion arranged predominantly in sheets, (H and E, ×40)

| Figure 2: Monotonous population of cells having scanty cytoplasm, round vesicular nucleus with finelygranular chromatin with marginated nucleoli, (H and E × 400)

| Figure 3: Pancytokeratin immunostaining showing cytoplasmic positivity and focal dot like positivity

| Figure 4: CK20 immunostaining shows characteristic perinuclear dot-like positivity
- 1 Schrama D, Ugurel S, Becker JC. Merkel cell carcinoma: Recent insights and new treatment options. Curr Opin Oncol 2012; 24: 141-9
- 2 Miller RW, Rabkin CS. Merkel cell carcinoma and melanoma: Etiological similarities and differences. Cancer Epidemiol Biomarkers Prev 1999; 8: 153-8
- 3 Heath M, Jaimes N, Lemos B, Mostaghimi A, Wang LC, Peñas PF. et al. Clinical characteristics of Merkel cell carcinoma at diagnosis in 195 patients: The AEIOU features. J Am Acad Dermatol 2008; 58: 375-81
- 4 Toker C. Trabecular carcinoma of the skin. Arch Dermatol 1972; 105: 107-10
- 5 De Wolff-Peeters C, Marien K, Mebis J, Desmet V. A cutaneous APUDoma or Merkel cell tumor? A morphologically recognizable tumor with a biological and histological malignant aspect in contrast with its clinical behavior. Cancer 1980; 46: 1810-6
- 6 Lyhne D, Lock-Andersen J, Dahlstrøm K, Drzewiecki KT, Balslev E, Muhic A. et al. Rising incidence of Merkel cell carcinoma. J Plast Surg Hand Surg 2011; 45: 274-80
- 7 Cirillo F, Vismarra M, Cafaro I, Martinotti M. Merkel cell carcinoma: A retrospective study on 48 cases and review of literature. J Oncol 2012; 2012: 749030
- 8 Feng H, Shuda M, Chang Y, Moore PS. Clonal integration of a polyomavirus in human merkel cell carcinoma. Science 2008; 319: 1096-100
- 9 DeCaprio JA. Merkel cell polyomavirus and Merkel cell carcinoma. Philos Trans R Soc Lond B Biol Sci 2017; 372: pii-20160276
- 10 Wong HH, Wang J. Merkel cell carcinoma. Arch Pathol Lab Med 2010; 134: 1711-6
- 11 Amin MB, Greene FL, Edge SB, Compton CC, Gershenwald JE, Brookland RK. et al. The eighth edition AJCC Cancer Staging Manual: continuing to build a bridge from a population-based to a more “personalized” approach to cancer staging. CA Cancer J Clin 2017; 67: 93-9
- 12 Yiengpruksawan A, Coit DG, Thaler HT, Urmacher C, Knapper WK. Merkel cell carcinoma. Prognosis and management. Arch Surg 1991; 126: 1514-9
- 13 Kritikos N, Priftakis D, Stavrinides S, Kleanthous S, Sarafianou E. Nuclear medicine techniques in merkel cell carcinoma: A case report and review of the literature. Oncol Lett 2015; 10: 1610-6
- 14 Strom T, Carr M, Zager JS, Naghavi A, Smith FO, Cruse CW. et al. Radiation therapy is associated with improved outcomes in Merkel cell carcinoma. Ann Surg Oncol 2016; 23: 3572-8
- 15 Chen MM, Roman SA, Sosa JA, Judson BL. The role of adjuvant therapy in the management of head and neck Merkel cell carcinoma: An analysis of 4815 patients. JAMA Otolaryngol Head Neck Surg 2015; 141: 137-41
- 16 Poulsen M, Rischin D, Walpole E, Harvey J, Mackintosh J, Ainslie J. et al. High-risk merkel cell carcinoma of the skin treated with synchronous carboplatin/etoposide and radiation: A Trans-Tasman Radiation Oncology group study – TROG 96:07. J Clin Oncol 2003; 21: 4371-6
- 17 Ito Y, Kawamura K, Miura T, Ueda K, Onodera H, Takahashi H. et al. Merkel cell carcinoma. A successful treatment with tumor necrosis factor. Arch Dermatol 1989; 125: 1093-5
- 18 Su LD, Fullen DR, Lowe L, Uherova P, Schnitzer B, Valdez R. et al. CD117 (KIT receptor) expression in merkel cell carcinoma. Am J Dermatopathol 2002; 24: 289-93
- 19 di Bartolomeo M, Bajetta E, Buzzoni R, Mariani L, Carnaghi C, Somma L. et al. Clinical efficacy of octreotide in the treatment of metastatic neuroendocrine tumors. A study by the Italian trials in medical oncology group. Cancer 1996; 77: 402-8
- 20 National Comprehensive Cancer Network. Clinical Practice Guideline Sinoncology. Available from: http://www.nccn.org/professionals/physician_gls/f_guidelines.asp. [Last accessed on 2019 July 25]
- 21 NCCN Clinical Practice Guidelines in Oncology. Merkel Cell Carcinoma. Ver. 1. 2019th ed. Fort Washington, PA: National Comprehensive Cancer Network, Inc.; 2019. Available from: https://www.nccn.org/professionals/physician_gls/pdf/mcc.pdf. [Last accessed on 2019 Oct 29].
- 22 Nghiem PT, Bhatia S, Lipson EJ, Kudchadkar RR, Miller NJ, Annamalai L. et al. PD-1 blockade with pembrolizumab in advanced Merkel-cell carcinoma. N Engl J Med 2016; 374: 2542-52
- 23 Kaufman HL, Russell JS, Hamid O, Bhatia S, Terheyden P, D’Angelo SP. et al. Updated efficacy of avelumab in patients with previously treated metastatic Merkel cell carcinoma after≥1 year of follow-up: JAVELIN Merkel 200, a phase 2 clinical trial. J Immunother Cancer 2018; 6: 7


 PDF
PDF  Views
Views  Share
Share

