Monoclonal Gammopathy in Chronic Lymphocytic Leukemia: A Case Report and Review of its Literature
CC BY-NC-ND 4.0 · Indian J Med Paediatr Oncol 2018; 39(02): 257-259
DOI: DOI: 10.4103/ijmpo.ijmpo_129_17
Abstract
The presence of monoclonal gammopathies in B-cell malignancies occurs frequently. Monoclonal proteins are present in a significant number of patients with chronic lymphocytic leukemia (CLL), which is a disorder of antigen-stimulated mature B-cells. The recognition of monoclonal proteins or light chains in the serum and/or urine is increased in the majority of CLL patients with the use of highly sensitive laboratory methods such as serum immunofixation studies. A different autoimmune phenomenon may explain the presence of some of these monoclonal proteins. Some reports indicate that the finding of monoclonal proteins has a negative impact on patients' survival. However, there is no clear evidence to suggest the prognostic significance of monoclonal proteins in patients with CLL. Although the presence of monoclonal proteins in CLL occurs usually at an incidence of 60%–80%, there are very few cases reported in literature. We report a case of CLL diagnosed in 2009 who developed disease progression along with the presence of immunoglobulin kappa monoclonal gammopathy. Although the presence of monoclonal gammopathy might be due to the use of highly sensitive methods, this can be due to autoimmune phenomenon or development from the same or different clone of B-cells.
Publication History
Article published online:
23 June 2021
© 2018. Indian Society of Medical and Paediatric Oncology. This is an open access article published by Thieme under the terms of the Creative Commons Attribution-NonDerivative-NonCommercial-License, permitting copying and reproduction so long as the original work is given appropriate credit. Contents may not be used for commercial purposes, or adapted, remixed, transformed or built upon. (https://creativecommons.org/licenses/by-nc-nd/4.0/.)
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India
Abstract
The presence of monoclonal gammopathies in B-cell malignancies occurs frequently. Monoclonal proteins are present in a significant number of patients with chronic lymphocytic leukemia (CLL), which is a disorder of antigen-stimulated mature B-cells. The recognition of monoclonal proteins or light chains in the serum and/or urine is increased in the majority of CLL patients with the use of highly sensitive laboratory methods such as serum immunofixation studies. A different autoimmune phenomenon may explain the presence of some of these monoclonal proteins. Some reports indicate that the finding of monoclonal proteins has a negative impact on patients' survival. However, there is no clear evidence to suggest the prognostic significance of monoclonal proteins in patients with CLL. Although the presence of monoclonal proteins in CLL occurs usually at an incidence of 60%–80%, there are very few cases reported in literature. We report a case of CLL diagnosed in 2009 who developed disease progression along with the presence of immunoglobulin kappa monoclonal gammopathy. Although the presence of monoclonal gammopathy might be due to the use of highly sensitive methods, this can be due to autoimmune phenomenon or development from the same or different clone of B-cells.
Introduction
Chronic lymphocytic leukemia (CLL) is considered as a disorder arising from antigen-stimulated mature B-cells by avoiding apoptosis. The presence of monoclonal proteins in association with CLL is less with conventional techniques and increases with the use of highly sensitive techniques. We report the case of a patient with CLL who developed monoclonal gammopathy associated with progressive disease in the form of anemia, thrombocytopenia, lymphadenopathy, and splenomegaly. Reports of gammopathy in CLL are very few. A total of three cases were reported in literature. The most recent case of biclonal gammopathy in CLL was reported in 2015 by Khalil.
Case Report
A 49-year-old male presented with left supraclavicular swelling on April 4, 2009. Clinical examination revealed palpable nontender left supraclavicular lymph node. Rest of the systemic examination was normal. His investigation revealed hemoglobin – 12.6 g/dl, leukocyte count – 18,300/cumm with lymphocytosis, and platelets – 126,000/μl. Lactate dehydrogenase and serum uric acid were normal. Total bilirubin was 0.7 mg/dl, serum glutamic oxaloacetic transaminase was 23 U/L, and serum glutamic pyruvic transaminase was 23 U/L. Direct and indirect Coombs test was negative. Flow cytometry revealed CD5, CD23, CD19, and CD20 positive. Bone marrow biopsy was suggestive of CLL with nodular, paratrabecular, and interstitial pattern. Karyotyping revealed 46XY in majority. Fluorescence in situ hybridization was normal in 87% of cells, heterogeneous deletion of 11q23 was observed in 8% of cells, Trisomy 12 was observed in 2% of cells, and deletion in 13q was observed in 7% of cells. Chest X-ray was normal. Ultrasound abdomen was suggestive of well-defined heterogeneous space-occupying lesion in segment IV of liver. Computed tomography of abdomen and pelvis showed segment 4a, 4b hypodense hepatic lesions with enhancement pattern, highly suggestive of hemangioma and mild splenomegaly. The patient was diagnosed as Chronic B-cell Lymphocytic leukemia Rai Stage - 0/1. He was on regular follow-up with complete blood counts and physical examination. He later presented on January 2017 with complaints of easy fatigability. Examination revealed presence of pallor, bilateral cervical, axillary lymphadenopathy, and massive splenomegaly (spleen palpable up to umbilicus). Investigations revealed hemoglobin –6.2gm/dl, leukocyte count –34,000/mm 3 with lymphocytosis and smudge cells in peripheral smear, and platelet count –80,000/μl. Serum quantitative immunofixation showed total immunoglobulin (IgG) to be 6190 mg/dl (normal: 700–1600), total IgA to be 18.80 mg/dl (normal: 70–400), and total IgM to be 11.40 mg/dl (normal: 40–230). Serum-free kappa (light chain) was 823 mg/L (normal: 3.3–19.4), Serum-free lambda was 6.86 mg/L (normal: 5.71–26.3), and free kappa lambda ratio was 119.62 (normal: 0.26–1.65). Beta-2 microglobulin was 3353 ng/ml. Serum protein electrophoresis revealed “M'' band of 4.85 gm%-in gamma region [Figure 1]. Urine for Bence–Jones protein was negative. Bone marrow biopsy revealed mature lymphocytes with immunohistochemistry positive for CD5, CD138, CD20, kappa, and lambda, suggesting CLL without any evidence of multiple myeloma [Figure 2]. Whole-body positron emission tomography scan revealed evidence of low-grade metabolically active disease in bilateral cervical, axillary, deep pectoral, abdominal, retroperitoneal, and bilateral pelvic lymph nodes and spleen and diffuse marrow uptake findings compatible with CLL [Figure 3]. Cytogenetics revealed deletion of p53 in 3%-cells, Trisomy 12 in 17%-cells, and deletion of 13q14 in 23%-cells [Figure 4]a,[Figure 4]b,[Figure 4]c. The patient was diagnosed with CLL modified Rai Stage IV with monoclonal IgG kappa gammopathy and he received 3 units of packed red blood cells and was started on tablet ibrutinib 140 mg thrice a day. After 2 weeks of ibrutinib treatment, the patient's lymph nodes and spleen clinically decreased in size and he was discharged in stable condition and advised for regular follow-up.
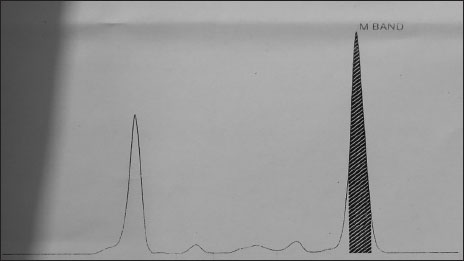
| Figure 1:Serum protein electrophoresis showing “M” band in gamma globulin region
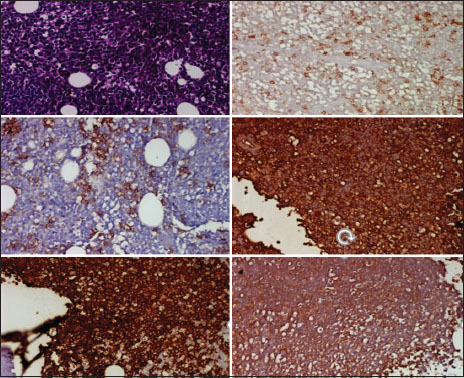
| Figure.2:Bone marrow biopsy showing mature lymphocytes with immunohistochemistry positive for CD5, CD138, CD20, kappa, and lambda, respectively
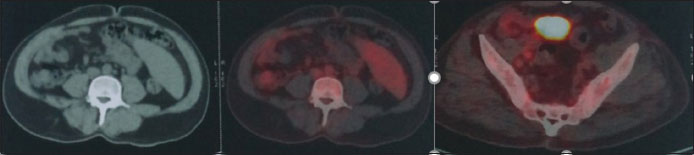
| Figure.5Positron emission tomography scan showing low-grade metabolically active abdominal, retroperitoneal, and bilateral pelvic lymph nodes and spleen and diffuse marrow uptake consistent with chronic lymphocytic leukemia

| Figure.4Fluorescence in situ hybridization in bone marrow aspirate showing (a) Two green and one orange signals indicating heterogeneous deletion of p53 in 3% of cells. (b) Three green, two orange, and two aqua signals indicating Trisomy 12 in 17% of cells. (c) Two green, one orange, and two aqua signals indicating heterogeneous deletion of 13q14.3 in 23% of cells
Discussion
CLL is associated with progressive hypogammaglobulinemia that can involve one or more IgG subclasses.[1] The incidence of monoclonal proteins in patients with CLL ranges between 60% and 80%.[2],[3] IgG kappa is the most common abnormality detected in CLL followed by IgG lambda, IgM kappa, IgM lambda, and IgA lambda in the order of frequency.[4] Free light chains are also commonly detected abnormalities in CLL. They can occur in 30%–40% of patients in CLL.[5] The exact pathophysiology of this monoclonal proteins is not known, it might be due to autoimmune phenomenon, derivation from same or different clone of B-cells. Some studies have shown that serum-free light chain abnormalities are associated with poor prognosis in CLL.[6],[7] The exact role of monoclonal or biclonal proteins in CLL is not clear, even though some studies have shown that the presence of monoclonal or biclonal proteins is a poor prognostic factor.[8] It is difficult to draw conclusions from previously reported cases regarding their role in CLL as there are few cases reported and as there are no randomized controlled studies done regarding this issue.
Conclusion
The clinical and prognostic significance of monoclonal proteins in CLL is not clear. More data, studies, and evidence are required for better understanding of its pathophysiology and use of this information in clinical practice for better outcomes. This may help us in therapeutic decision-making in the future.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Conflict of Interest
There are no conflicts of interest.
References
- Baliakas P, Xochelli A, Minga E, Hadzidimitriou A, Douka V, Karavalakis G. et al. Revisiting hypogammaglobulinemia in chronic lymphocytic leukemia: A combined clinicobiological approach. Blood 2014; 124: 5633
- Deegan MJ, Abraham JP, Sawdyk M, Van Slyck EJ. High incidence of monoclonal proteins in the serum and urine of chronic lymphocytic leukemia patients. Blood 1984; 64: 1207-11
- Beaume A, Brizard A, Dreyfus B, Preud'homme JL. High incidence of serum monoclonal Igs detected by a sensitive immunoblotting technique in B-cell chronic lymphocytic leukemia. Blood 1994; 84: 1216-9
- Yegin ZA, Ozkurt ZN, Yaǧci M. Free light chain: A novel predictor of adverse outcome in chronic lymphocytic leukemia. Eur J Haematol 2010; 84: 406-11
- Noel P, Kyle RA. Monoclonal proteins in chronic lymphocytic leukemia. Am J Clin Pathol 1987; 87: 385-8
- Maurer MJ, Cerhan JR, Katzmann JA, Link BK, Allmer C, Zent CS. et al. Monoclonal and polyclonal serum free light chains and clinical outcome in chronic lymphocytic leukemia. Blood 2011; 118: 2821-6
- Pratt G, Harding S, Holder R, Fegan C, Pepper C, Oscier D. et al. Abnormal serum free light chain ratios are associated with poor survival and may reflect biological subgroups in patients with chronic lymphocytic leukaemia. Br J Haematol 2009; 144: 217-22
- Bernstein ZP, Fitzpatrick JE, O'Donnell A, Han T, Foon KA, Bhargava A. et al Clinical significance of monoclonal proteins in chronic lymphocytic leukemia. Leukemia 1992; 6: 1243-5
Address for correspondence
Publication History
Article published online:
23 June 2021
© 2018. Indian Society of Medical and Paediatric Oncology. This is an open access article published by Thieme under the terms of the Creative Commons Attribution-NonDerivative-NonCommercial-License, permitting copying and reproduction so long as the original work is given appropriate credit. Contents may not be used for commercial purposes, or adapted, remixed, transformed or built upon. (https://creativecommons.org/licenses/by-nc-nd/4.0/.)
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2, Noida-201301 UP,
India

| Figure 1:Serum protein electrophoresis showing “M” band in gamma globulin region

| Figure.2:Bone marrow biopsy showing mature lymphocytes with immunohistochemistry positive for CD5, CD138, CD20, kappa, and lambda, respectively

| Figure.5Positron emission tomography scan showing low-grade metabolically active abdominal, retroperitoneal, and bilateral pelvic lymph nodes and spleen and diffuse marrow uptake consistent with chronic lymphocytic leukemia

| Figure.4Fluorescence in situ hybridization in bone marrow aspirate showing (a) Two green and one orange signals indicating heterogeneous deletion of p53 in 3% of cells. (b) Three green, two orange, and two aqua signals indicating Trisomy 12 in 17% of cells. (c) Two green, one orange, and two aqua signals indicating heterogeneous deletion of 13q14.3 in 23% of cells
References
- Baliakas P, Xochelli A, Minga E, Hadzidimitriou A, Douka V, Karavalakis G. et al. Revisiting hypogammaglobulinemia in chronic lymphocytic leukemia: A combined clinicobiological approach. Blood 2014; 124: 5633
- Deegan MJ, Abraham JP, Sawdyk M, Van Slyck EJ. High incidence of monoclonal proteins in the serum and urine of chronic lymphocytic leukemia patients. Blood 1984; 64: 1207-11
- Beaume A, Brizard A, Dreyfus B, Preud'homme JL. High incidence of serum monoclonal Igs detected by a sensitive immunoblotting technique in B-cell chronic lymphocytic leukemia. Blood 1994; 84: 1216-9
- Yegin ZA, Ozkurt ZN, Yaǧci M. Free light chain: A novel predictor of adverse outcome in chronic lymphocytic leukemia. Eur J Haematol 2010; 84: 406-11
- Noel P, Kyle RA. Monoclonal proteins in chronic lymphocytic leukemia. Am J Clin Pathol 1987; 87: 385-8
- Maurer MJ, Cerhan JR, Katzmann JA, Link BK, Allmer C, Zent CS. et al. Monoclonal and polyclonal serum free light chains and clinical outcome in chronic lymphocytic leukemia. Blood 2011; 118: 2821-6
- Pratt G, Harding S, Holder R, Fegan C, Pepper C, Oscier D. et al. Abnormal serum free light chain ratios are associated with poor survival and may reflect biological subgroups in patients with chronic lymphocytic leukaemia. Br J Haematol 2009; 144: 217-22
- Bernstein ZP, Fitzpatrick JE, O'Donnell A, Han T, Foon KA, Bhargava A. et al Clinical significance of monoclonal proteins in chronic lymphocytic leukemia. Leukemia 1992; 6: 1243-5


 PDF
PDF  Views
Views  Share
Share

