Morphometric evaluation and clinical correlations in pediatric malignant small round cell tumors
CC BY-NC-ND 4.0 · Indian J Med Paediatr Oncol 2014; 35(04): 267-270
DOI: DOI: 10.4103/0971-5851.144987
Abstract
Aims: Nuclear size increases in malignant tumors and reflects DNA content, ploidy and proliferation index. Present study investigated if the nuclear morphometry could differentiate histomorphologically similar paediatric malignant small round cell tumors on hematoxylin and eosin stained sections for diagnostics in a resource poor setting. Settings and Design: All the consecutive malignant pediatric tumors received in Pathology Department from other faculties of King George′s Medical University and also those referred directly to Pathology Department from other hospitals of city/other cities during 3 years period were recorded. Materials and Methods: Morphometric analysis was done in 22 confirmed (by higher ancillary techniques) but histomorphologically difficult to differentiate round cell tumors. All sections were analyzed by cell images from six different areas, using Leica Q win 500 images software. Results: Nuclear measurements were obtained for retinoblastoma (RB) (nine cases), neuroblastoma (five cases), Wilms tumor (WT) (three cases), rhabdomyosarcoma (three cases), malignant hemangiopericytoma (one case) and non-Hodgkin lymphoma (one case). Among the RBs, maximum mean nuclear area percent (24.93) was seen in a case with nerve involvement and metastasis, followed by cases with only nerve involvement (21.60) and smallest area (16.57) was in non-nerve involving, nonmetastatic cases. All five cases of neuroblastoma had almost similar mean nuclear area percent (18.05-18.29). WT case with metastasis had higher nuclear area (21.25) than nonmetastatic (19.47). Amongst all the tumors, minimum value (14.93) was seen in malignant hemangiopericytoma. Conclusion: Morphometric evaluation in paediatric malignant round cell tumors have generated useful data, and needs further multicentric confirmation for implementation.
Publication History
Article published online:
19 July 2021
© 2014. Indian Society of Medical and Paediatric Oncology. This is an open access article published by Thieme under the terms of the Creative Commons Attribution-NonDerivative-NonCommercial-License, permitting copying and reproduction so long as the original work is given appropriate credit. Contents may not be used for commercial purposes, or adapted, remixed, transformed or built upon. (https://creativecommons.org/licenses/by-nc-nd/4.0/.)
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India
Abstract
Aims:
Nuclear size increases in malignant tumors and reflects DNA content, ploidy and proliferation index. Present study investigated if the nuclear morphometry could differentiate histomorphologically similar paediatric malignant small round cell tumors on hematoxylin and eosin stained sections for diagnostics in a resource poor setting.
Settings and Design:
All the consecutive malignant pediatric tumors received in Pathology Department from other faculties of King George's Medical University and also those referred directly to Pathology Department from other hospitals of city/other cities during 3 years period were recorded.
Materials and Methods:
Morphometric analysis was done in 22 confirmed (by higher ancillary techniques) but histomorphologically difficult to differentiate round cell tumors. All sections were analyzed by cell images from six different areas, using Leica Q win 500 images software.
Results:
Nuclear measurements were obtained for retinoblastoma (RB) (nine cases), neuroblastoma (five cases), Wilms tumor (WT) (three cases), rhabdomyosarcoma (three cases), malignant hemangiopericytoma (one case) and non-Hodgkin lymphoma (one case). Among the RBs, maximum mean nuclear area percent (24.93) was seen in a case with nerve involvement and metastasis, followed by cases with only nerve involvement (21.60) and smallest area (16.57) was in non-nerve involving, nonmetastatic cases. All five cases of neuroblastoma had almost similar mean nuclear area percent (18.05-18.29). WT case with metastasis had higher nuclear area (21.25) than nonmetastatic (19.47). Amongst all the tumors, minimum value (14.93) was seen in malignant hemangiopericytoma.
Conclusion:
Morphometric evaluation in paediatric malignant round cell tumors have generated useful data, and needs further multicentric confirmation for implementation.
INTRODUCTION
Small round cell tumors (SRCTs) are characterized both cytologically and histologically by predominantly small, round to oval and relatively undifferentiated cells. They constitute an overwhelming majority of childhood malignancies. This group of malignancies include retinoblastoma (RB), neuroblastoma, hepatoblastoma, nephroblastoma, rhabdomyosarcoma (RMS), small cell anaplastic carcinoma, Ewing sarcoma peripheral neuroectodermal tumor, desmoplastic SRCT and non-Hodgkin lymphoma (NHL). SRCTs often pose a diagnostic challenge because of the morphologic similarities, especially when they are poorly differentiated.[1,2,3,4,5] To cut short the differential diagnoses list, and to arrive at a definite diagnosis, diagnostic accuracy of SRCTs can be enhanced along with one or more of the ancillary techniques[6] such as special stains (cytochemistry), immunocytochemistry,[7,8] electron microscopy,[9] morphometry,[10] tissue culture, DNA ploidy, karyotype and molecular analysis.[11,12,13] In the present study, we have worked out the role of nuclear morphometry to differentiate histomorphologically similar paediatric malignant SRCTs in hematoxylin and eosin (H and E) stained sections for using in a poor resource country. Majority of histopathology laboratories of a developing country do not imply auxiliary techniques, so the present study investigated the feasibility of precise diagnosis based on histomorphology and morphometry.
MATERIALS AND METHODS
All the consecutive malignant pediatric tumors received in Pathology Department from other faculties of King George's Medical University and also those referred directly to Pathology Department from other hospitals of city/other cities during 3 years period were recorded. Out of a total of 83 cases, 22 cases were selected to perform morphometric analysis. These 22 cases of SRCT elude identification on light microscopy (H and E stained sections) alone. Histologically, tissue sections showed relatively undifferentiated cells which were predominantly small round to oval. These archived cases were already worked up cases with application of special stains (cytochemistry), immunohistochemistry and molecular techniques as per the individual case diagnostic requirement. Morphometric analysis was done on representative areas of H and E sections with Leica Q Win 500 image analysis software (Leica GMBH, West Germany). For a single case, images were captured from a minimum of six different areas. Nuclei were tagged blue and with Acquisition and Analysis software; parameters such as nuclear area, nuclear diameter, nuclear perimeter, area fraction, area fill count, percent area etc., were calculated. Final results were obtained from the mean of percent areas of different images for a particular case and results were compared and analysed.
RESULTS
Patient age varied from 6 months to 13 years with a mean age of 6 years. Male children (16) were more than female (6). Morphometric analysis was obtained for RB (nine cases), neuroblastoma (six cases), Wilms tumor (WT) (three cases), RMS (three cases), malignant hemangiopericytoma (one case) and NHL (one case). Nuclear area percent varied from 14.93 to 24.93 with a mean value of 18.91. Amongst the RBs, maximum mean nuclear area percent (24.93) was seen in a case with nerve involvement and metastasis, followed by cases with only nerve involvement (21.60) and smallest area (16.57) was in non-nerve involving, nonmetastatic case. Three cases of RB with optic nerve involvement (case number 5, 8, 9) showed significant increase in mean nuclear area percent. Neuroblastoma cases (5) had similar results for mean nuclear area ranging from 18.05 to 18.29. WT case with metastasis had maximum nuclear area (21.25) than nonmetastatic (19.47). Case number 16 was diagnosed as poorly differentiated WT with blastema predominance, but morphometry yielded results in almost similar range. Embryonal RMS nuclear area (19.29) was more than alveolar RMS (18.00). Amongst all the tumors, minimum value (14.93) was seen in malignant hemangiopericytoma. Only one case was of NHL and no definite conclusion could be withdrawn to compare it with. Individual case diagnosis with details of age, sex and morhometric analysis is summarised in Table 1.
Table 1
Results of morphometry in 22 cases of pediatric SRCTs
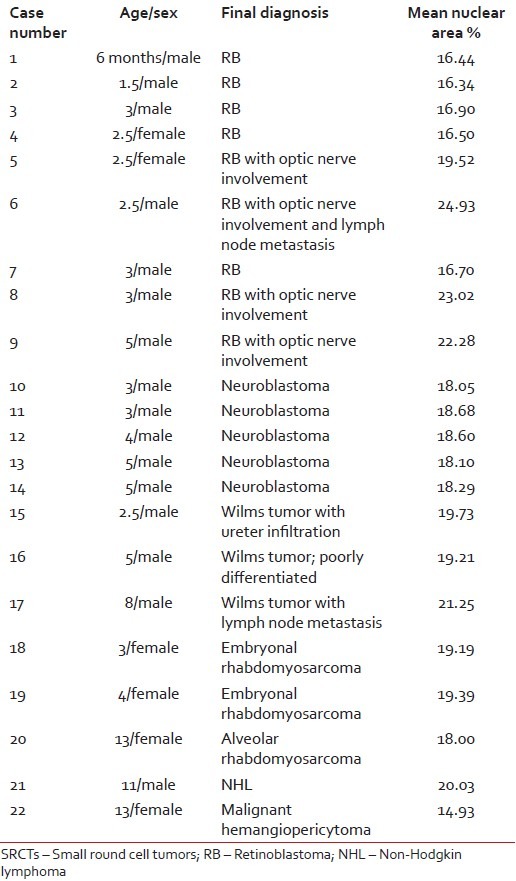
|
DISCUSSION
Morphometry is a type of quantitative analysis[14,15,16,17] and have been applied for a number of varied sites like breast, gastrointestinal tract, acute leukemias etc.[18,19,20] Childhood malignancies are rare, but those that occur often have the appearance of primitive “small round blue cell tumors” and pose a diagnostic challenge to the pathologist. Accurate tissue diagnosis is imperative in the group of tumors lumped under the category of malignant SRCT, because the treatment and management of these tumors is different.[21]
In the present study, malignant SRCTs in pediatric population irrespective of the region involved were included. Male children outnumbered female (16:6) and majority cases were of RB (40.9%) followed by neuroblastoma (22.7%). Morphometric analysis on SRCTs by Brahmi et al. included Ewing's sarcoma (32.7%) and neuroblastoma (18%).[10] Ravindra and Kini studied morphometry of SRCTs in the region of the kidney and found WT (58%) was in majority with neuroblastoma (27.7%) being the second most common.[22] Kazanowska et al. studied in particular childhood RMS with maximum cases of embryonal RMS (60.2%) and concluded nuclear morphometry is a useful tool in the assessment of children with RMS.[23]
The available literature reveals that most of the researchers who worked on SRCTs have applied morphometry on aspiration smears,[10,20] investigated a particular differential of SRCT[22,23,24,25,26] and have also included adult subjects in addition to pediatric subjects.[10,22,23] Studies considering SRCTs in only pediatric subjects and applying morphometry on histopathology sections are few.[18,19,24,25,27]
The present study had the highest maximum mean nuclear area percent in a case of RB with nerve involvement and metastasis (24.93) and cases with nerve involvement had higher values than non-nerve involving. Brahmi et al. concluded that RB group of tumors showed the highest mean nuclear area and RB could successfully be differentiated from all other Materials Research Centers and Teams (MRCTs) with the help of morphometry.[10] Intratumoral heterogeneity of MYCN in neuroblastomas is rare.[28] Though, from our data we found that mean nuclear area percent was higher in cases of nerve involvement and metastasis but taking into consideration the overall mean values of RB, no significant difference was found from other categories.
We observed embryonal RMS nuclear area (19.29) was more than alveolar RMS (18.00). Brahmi et al. concluded that RMS had the highest mean convex area and RMS could be differentiated from all other MRCTs with the help of morphometry.[10]
CONCLUSION
In the present study, morphometric evaluation of pediatric SRCTs show that within a tumor type, the mean nuclear area percentage increases with tumor aggressiveness (i.e., lymphnode metastasis or nerve invasion). However, the evaluation did not generate any specific mean nuclear area range which could classify and categorize the differentials of SRCTs. Morphometric analysis in pediatric malignant SRCTs needs further multicentric confirmation studies. These studies can throw light on the categorization and prognosis of tumors provided the evaluation is done at specialized centres where specific tumors of one type are encountered, treated and followed-up.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- Variend S. Small cell tumours in childhood: A review. J Pathol 1985;145:1-25.
- Carter RL, McCarthy KP. Divergent differentiation in round-cell tumours of soft tissues: An interim appraisal. Histopathology 1993;23:93-7.
- Kocjan G. Diagnostic dilemmas in FNAC cytology: Small round cell tumors. In: Schroder G, editor. Fine needle aspiration cytology diagnostic principles and dilemmas. Berlin: Springer-Verlag; 2006. p. 133-4.
- Akhtar M, Iqbal MA, Mourad W, Ali MA. Fine-needle aspiration biopsy diagnosis of small round cell tumors of childhood: A comprehensive approach. Diagn Cytopathol 1999;21:81-91.
- Radhika S, Bakshi A, Rajwanshi A, Nijhawan R, Das A, Kakkar N, et al. Cytopathology of uncommon malignant renal neoplasms in the pediatric age group. Diagn Cytopathol 2005;32:281-6.
- Saleh H, Masood S. Value of ancillary studies in fine-needle aspiration biopsy. Diagn Cytopathol 1995;13:310-5.
- Brahmi U, Rajwanshi A, Joshi K, Dey P, Vohra H, Ganguly NK, et al. Flow cytometric immunophenotyping and comparison with immunocytochemistry in small round cell tumors. Anal Quant Cytol Histol 2001;23:405-12.
- Leon ME, Hou JS, Galindo LM, Garcia FU. Fine-needle aspiration of adult small-round-cell tumors studied with flow cytometry. Diagn Cytopathol 2004;31:147-54.
- Akhtar M, Ali MA, Sabbah R, Bakry M, Nash JE. Fine-needle aspiration biopsy diagnosis of round cell malignant tumors of childhood. A combined light and electron microscopic approach. Cancer 1985;55:1805-17.
- Brahmi U, Rajwanshi A, Joshi K, Dey P, Vohra H, Ganguly NK, et al. Automated nuclear image morphometry on fine needle aspiration smears of malignant round cell tumors. Anal Quant Cytol Histol 2001;23:287-90.
- Bayani J, Thorner P, Zielenska M, Pandita A, Beatty B, Squire JA. Application of a simplified comparative genomic hybridization technique to screen for gene amplification in pediatric solid tumors. Pediatr Pathol Lab Med 1995;15:831-44.
- Peter M, Gilbert E, Delattre O. A multiplex real-time PCR assay for the detection of gene fusions observed in solid tumors. Lab Invest 2001;81:905-12.
- Thorner PS, Squire JA. Molecular genetics in the diagnosis and prognosis of solid pediatric tumors. Pediatr Dev Pathol 1998;1:337-65.
- Collan Y. Morphometry in pathology: Another look at diagnostic histopathology. Pathol Res Pract 1984;179:189-92.
- Marchevsky AM, Gil J. Applications of computerized interactive morphometry in pathology. II. A model for computer generated diagnosis. Lab Invest 1986;54:708-16.
- Rahman SM, Itakura H. Morphometry in histopathology. An image analysis workstation for the pathology laboratory. Anal Quant Cytol Histol 1996;18:471-80.
- Hamilton PW, Allen DC. Morphometry in histopathology. J Pathol 1995;175:369-79.
- Søreide K, Nedrebø BS, Reite A, Thorsen K, Kørner H. Endoscopy, morphology, morphometry and molecular markers: Predicting cancer risk in colorectal adenoma. Expert Rev Mol Diagn 2009;9:125-37.
- Tan PH, Goh BB, Chiang G, Bay BH. Correlation of nuclear morphometry with pathologic parameters in ductal carcinoma in situ of the breast. Mod Pathol 2001;14:937-41.
- Rajesh L, Pattari SK, Garewal G, Dey P, Srinivasan R. Image morphometry of acute leukemias. Comparison between lymphoid and myeloid subtypes. Anal Quant Cytol Histol 2004;26:57-60.
- Gautam U, Srinivasan R, Rajwanshi A, Bansal D, Marwaha RK. Comparative evaluation of flow-cytometric immunophenotyping and immunocytochemistry in the categorization of malignant small round cell tumors in fine-needle aspiration cytologic specimens. Cancer 2008;114:494-503.
- Ravindra S, Kini U. Cytomorphology and morphometry of small round-cell tumors in the region of the kidney. Diagn Cytopathol 2005;32:211-6.
- Kazanowska B, Jelen M, Reich A, Tarnawski W, Chybicka A. The role of nuclear morphometry in prediction of prognosis for rhabdomyosarcoma in children. Histopathology 2004;45:352-9.
- Oda Y, Tsuneyoshi M. A comparative study of nuclear morphometry and proliferating activity in neuroectodermal tumors of bone and Ewing′s sarcoma of bone. Gen Diagn Pathol 1995;141:121-9.
- Machado I, Ruiz-Sauri A, López-Guerrero JA, Llombart-Bosch A. Morphometric analysis of DNA ploidy and nuclear cell cycle regulators in Ewing′s sarcoma/primitive neuroectodermal tumor. Anal Quant Cytol Histol 2011;33:101-10.
- Callaghan RC, Carda C, Peydró-Olaya A, Triche T, Llombart-Bosch A. Small round cell tumors of bone and soft tissue. A morphometric and stereometric comparative analysis of 119 cases. Anal Quant Cytol Histol 1995;17:374-82.
- Ushigome S, Shimoda T, Nikaido T, Nakamori K, Miyazawa Y, Shishikura A, et al. Primitive neuroectodermal tumors of bone and soft tissue. With reference to histologic differentiation in primary or metastatic foci. Acta Pathol Jpn 1992;42:483-93.
- Theissen J, Boensch M, Spitz R, Betts D, Stegmaier S, Christiansen H, et al. Heterogeneity of the MYCN oncogene in neuroblastoma. Clin Cancer Res 2009;15:2085-90.
References
- Variend S. Small cell tumours in childhood: A review. J Pathol 1985;145:1-25.
- Carter RL, McCarthy KP. Divergent differentiation in round-cell tumours of soft tissues: An interim appraisal. Histopathology 1993;23:93-7.
- Kocjan G. Diagnostic dilemmas in FNAC cytology: Small round cell tumors. In: Schroder G, editor. Fine needle aspiration cytology diagnostic principles and dilemmas. Berlin: Springer-Verlag; 2006. p. 133-4.
- Akhtar M, Iqbal MA, Mourad W, Ali MA. Fine-needle aspiration biopsy diagnosis of small round cell tumors of childhood: A comprehensive approach. Diagn Cytopathol 1999;21:81-91.
- Radhika S, Bakshi A, Rajwanshi A, Nijhawan R, Das A, Kakkar N, et al. Cytopathology of uncommon malignant renal neoplasms in the pediatric age group. Diagn Cytopathol 2005;32:281-6.
- Saleh H, Masood S. Value of ancillary studies in fine-needle aspiration biopsy. Diagn Cytopathol 1995;13:310-5.
- Brahmi U, Rajwanshi A, Joshi K, Dey P, Vohra H, Ganguly NK, et al. Flow cytometric immunophenotyping and comparison with immunocytochemistry in small round cell tumors. Anal Quant Cytol Histol 2001;23:405-12.
- Leon ME, Hou JS, Galindo LM, Garcia FU. Fine-needle aspiration of adult small-round-cell tumors studied with flow cytometry. Diagn Cytopathol 2004;31:147-54.
- Akhtar M, Ali MA, Sabbah R, Bakry M, Nash JE. Fine-needle aspiration biopsy diagnosis of round cell malignant tumors of childhood. A combined light and electron microscopic approach. Cancer 1985;55:1805-17.
- Brahmi U, Rajwanshi A, Joshi K, Dey P, Vohra H, Ganguly NK, et al. Automated nuclear image morphometry on fine needle aspiration smears of malignant round cell tumors. Anal Quant Cytol Histol 2001;23:287-90.
- Bayani J, Thorner P, Zielenska M, Pandita A, Beatty B, Squire JA. Application of a simplified comparative genomic hybridization technique to screen for gene amplification in pediatric solid tumors. Pediatr Pathol Lab Med 1995;15:831-44.
- Peter M, Gilbert E, Delattre O. A multiplex real-time PCR assay for the detection of gene fusions observed in solid tumors. Lab Invest 2001;81:905-12.
- Thorner PS, Squire JA. Molecular genetics in the diagnosis and prognosis of solid pediatric tumors. Pediatr Dev Pathol 1998;1:337-65.
- Collan Y. Morphometry in pathology: Another look at diagnostic histopathology. Pathol Res Pract 1984;179:189-92.
- Marchevsky AM, Gil J. Applications of computerized interactive morphometry in pathology. II. A model for computer generated diagnosis. Lab Invest 1986;54:708-16.
- Rahman SM, Itakura H. Morphometry in histopathology. An image analysis workstation for the pathology laboratory. Anal Quant Cytol Histol 1996;18:471-80.
- Hamilton PW, Allen DC. Morphometry in histopathology. J Pathol 1995;175:369-79.
- Søreide K, Nedrebø BS, Reite A, Thorsen K, Kørner H. Endoscopy, morphology, morphometry and molecular markers: Predicting cancer risk in colorectal adenoma. Expert Rev Mol Diagn 2009;9:125-37.
- Tan PH, Goh BB, Chiang G, Bay BH. Correlation of nuclear morphometry with pathologic parameters in ductal carcinoma in situ of the breast. Mod Pathol 2001;14:937-41.
- Rajesh L, Pattari SK, Garewal G, Dey P, Srinivasan R. Image morphometry of acute leukemias. Comparison between lymphoid and myeloid subtypes. Anal Quant Cytol Histol 2004;26:57-60.
- Gautam U, Srinivasan R, Rajwanshi A, Bansal D, Marwaha RK. Comparative evaluation of flow-cytometric immunophenotyping and immunocytochemistry in the categorization of malignant small round cell tumors in fine-needle aspiration cytologic specimens. Cancer 2008;114:494-503.
- Ravindra S, Kini U. Cytomorphology and morphometry of small round-cell tumors in the region of the kidney. Diagn Cytopathol 2005;32:211-6.
- Kazanowska B, Jelen M, Reich A, Tarnawski W, Chybicka A. The role of nuclear morphometry in prediction of prognosis for rhabdomyosarcoma in children. Histopathology 2004;45:352-9.
- Oda Y, Tsuneyoshi M. A comparative study of nuclear morphometry and proliferating activity in neuroectodermal tumors of bone and Ewing′s sarcoma of bone. Gen Diagn Pathol 1995;141:121-9.
- Machado I, Ruiz-Sauri A, López-Guerrero JA, Llombart-Bosch A. Morphometric analysis of DNA ploidy and nuclear cell cycle regulators in Ewing′s sarcoma/primitive neuroectodermal tumor. Anal Quant Cytol Histol 2011;33:101-10.
- Callaghan RC, Carda C, Peydró-Olaya A, Triche T, Llombart-Bosch A. Small round cell tumors of bone and soft tissue. A morphometric and stereometric comparative analysis of 119 cases. Anal Quant Cytol Histol 1995;17:374-82.
- Ushigome S, Shimoda T, Nikaido T, Nakamori K, Miyazawa Y, Shishikura A, et al. Primitive neuroectodermal tumors of bone and soft tissue. With reference to histologic differentiation in primary or metastatic foci. Acta Pathol Jpn 1992;42:483-93.
- Theissen J, Boensch M, Spitz R, Betts D, Stegmaier S, Christiansen H, et al. Heterogeneity of the MYCN oncogene in neuroblastoma. Clin Cancer Res 2009;15:2085-90.


 PDF
PDF  Views
Views  Share
Share

