Needle core biopsy for breast lesions: An audit of 467 needle core biopsies
CC BY-NC-ND 4.0 · Indian J Med Paediatr Oncol 2013; 34(04): 252-256
DOI: DOI: 10.4103/0971-5851.125237
Abstract
Background: Breast cancer is the commonest cancer among women in urban India. Triple assessment includes clinical, radiological and cytological assessment of breast lesions. Guided core needle biopsy has replaced fine needle aspiration cytology in most of the western countries. In resource poor countries FNAC is still a very valuable and cost effective method to diagnose breast lesions. Pitfalls include increased rates of non diagnostic smears, and inadequate smears. Further procedures may be required and this increases the cost, anxiety and delay in diagnosis. Aims: The aim of this study is to analyze the concordance of radiological and histopathology findings in BI-RADS category 3,4,5 lesions following a core biopsy. Materials and Methods: Data was retrospectively collected from consecutive symptomatic and opportunistic screen detected patients with abnormalities who underwent ultrasound guided interventional procedures from Jan 2010 to Aug 2011. Symptomatic patients underwent clinical examination, mammogram and breast ultrasound. Women under 35 years of age had only breast ultrasound. Core biopsy was performed under ultrasound guidance or clinically by a breast surgeon/ radiologist for BI-RADS category 3,4,5 lesions. Statistical Methods: Chi square test was done to show the strength of association of imaging findings and histopathology results of core biopsy. Results: 437 patients were symptomatic and 30 patients had screen detected abnormalities. The positive predictive value for BI-RADS 5 lesions for malignancy is 93.25% and the negative predictive value of BI-RADS category 3 lesions for cancer is 98.4%. False negative diagnosis on core biopsy was 0.85%. We were able to defer surgery in 60% of the patients with a clear radiological and pathological benign diagnosis. Conclusion: The PPV and NPV for cancer is high with needle core biopsy in BI-RADS 3,4,5 lesions. Where there is no discordance between clinical, radiology and pathology findings, surgery can be avoided in benign lesions. While in resource poor countries FNAC continues to be a valuable method in the diagnosis of palpable and non palpable breast lesions, the practice of needle core biopsy provides the most accurate and optimal diagnostic information.
Publication History
Article published online:
19 July 2021
© 2013. Indian Society of Medical and Paediatric Oncology. This is an open access article published by Thieme under the terms of the Creative Commons Attribution-NonDerivative-NonCommercial-License, permitting copying and reproduction so long as the original work is given appropriate credit. Contents may not be used for commercial purposes, or adapted, remixed, transformed or built upon. (https://creativecommons.org/licenses/by-nc-nd/4.0/.)
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India
Abstract
Background:
Breast cancer is the commonest cancer among women in urban India. Triple assessment includes clinical, radiological and cytological assessment of breast lesions. Guided core needle biopsy has replaced fine needle aspiration cytology in most of the western countries. In resource poor countries FNAC is still a very valuable and cost effective method to diagnose breast lesions. Pitfalls include increased rates of non diagnostic smears, and inadequate smears. Further procedures may be required and this increases the cost, anxiety and delay in diagnosis.
Aims:
The aim of this study is to analyze the concordance of radiological and histopathology findings in BI-RADS category 3,4,5 lesions following a core biopsy.
Materials and Methods:
Data was retrospectively collected from consecutive symptomatic and opportunistic screen detected patients with abnormalities who underwent ultrasound guided interventional procedures from Jan 2010 to Aug 2011. Symptomatic patients underwent clinical examination, mammogram and breast ultrasound. Women under 35 years of age had only breast ultrasound. Core biopsy was performed under ultrasound guidance or clinically by a breast surgeon/ radiologist for BI-RADS category 3,4,5 lesions.
Statistical Methods:
Chi square test was done to show the strength of association of imaging findings and histopathology results of core biopsy.
Results:
437 patients were symptomatic and 30 patients had screen detected abnormalities. The positive predictive value for BI-RADS 5 lesions for malignancy is 93.25% and the negative predictive value of BI-RADS category 3 lesions for cancer is 98.4%. False negative diagnosis on core biopsy was 0.85%. We were able to defer surgery in 60% of the patients with a clear radiological and pathological benign diagnosis.
Conclusion:
The PPV and NPV for cancer is high with needle core biopsy in BI-RADS 3,4,5 lesions. Where there is no discordance between clinical, radiology and pathology findings, surgery can be avoided in benign lesions. While in resource poor countries FNAC continues to be a valuable method in the diagnosis of palpable and non palpable breast lesions, the practice of needle core biopsy provides the most accurate and optimal diagnostic information.
INTRODUCTION
Breast cancer is the most common cancer diagnosed in women world-wide accounting for 21% of all cancers diagnosed in women. Breast cancer incidence in India is increasing and has now become the most common cancer among women, surpassing cervical cancer in all the urban cancer registries.
Pre-operative pathology diagnosis constitutes an essential part of the work-up of breast lesions. In India, fine needle aspiration cytology (FNAC) is still widely practiced in the assessment of breast masses in both palpable and non-palpable lesions because it provides a rapid, accurate and cost-effective diagnosis. However, there are many pitfalls with FNAC in the assessment of breast lesions, leading to too many excision biopsies for diagnosis of breast masses.[1]
Core biopsy has replaced fine needle aspiration for symptomatic and screen detected breast lesions in most of the western countries. The frequency of non-diagnostic or inadequate sample report is lower than that of FNAC and it is much less invasive and less expensive when compared with excision or incision biopsy for diagnosis.
This is a retrospective study analyzing the results of 467 patients who underwent needle core biopsy of breast imaging reporting and data system (BI-RADS) –3, 4 and 5 lesions.
AIM
The aim of this retrospective analysis is to study the concordance of radiological and histopathological findings in BI-RADS category 3, 4 and 5 lesions.
MATERIALS AND METHODS
This is a single Institution retrospective study from January 2010 to August 2011. Data was retrospectively collected from consecutive symptomatic and opportunistic screen detected patients with abnormalities who underwent ultrasound guided interventional procedures. The age in this study ranged from 28 to 76 years. Symptomatic patients underwent clinical examination, mammogram and breast ultrasound. Women under 35 years of age had only breast ultrasound. Women who came for opportunistic screening had a mammogram after 40 years and had correlated ultrasound when required. Women who had BI-RADS -3, 4 or 5 lesions on ultrasound (both palpable and non-palpable) were recommended to undergo a core biopsy for definitive histopathological correlation. A total of 437 patients were symptomatic and 30 patients had screen detected abnormalities.
Core biopsy was performed under ultrasound guidance or clinically by a breast surgeon/radiologist. It is our practice to take a verbal consent from patient prior to the procedure. Routine tests to study coagulation parameters were not carried out before the biopsy unless patient was on warfarin. Core biopsy was performed using a 14G automated biopsy gun under local anesthesia with 2% plain lignocaine. Two to three cores were taken and fixed in buffered formalin and all were processed and read by a team of pathologists from a single institution. Pressure was applied locally for 5 min to stop bleeding. Antibiotics were not routinely used post-procedure. Simple analgesics were advised for 2 days post-procedure. Documentation of the biopsy always included an image with the needle in the lesion [Figure 1].
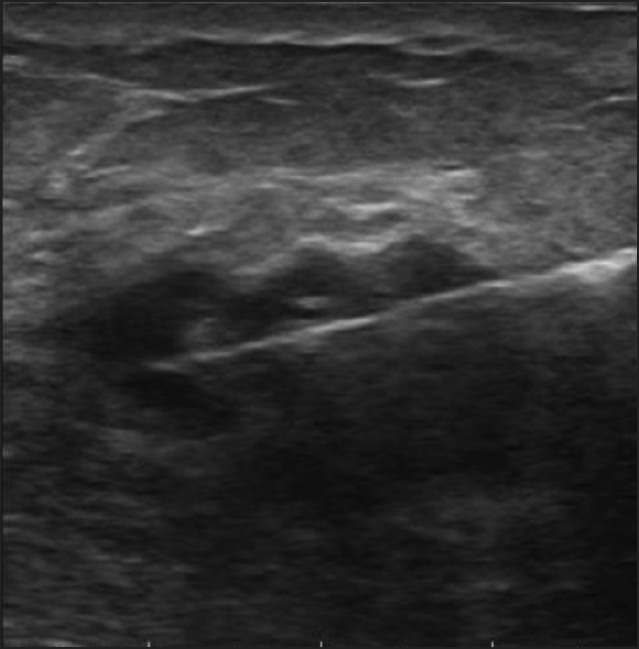
| Fig. 1 Image showing core needle within the lesion
Malignant lesions on histopathology underwent staging work-up as deemed necessary and followed-up with appropriate treatment. Patients with radiological and histopathological discordance were recommended surgical excision. Patients with a clear benign diagnosis on histopathology were given the options of follow-up and excision based on their age, clinical findings and radiological criteria.
Chi-square (χ2) test was done to show the strength of association of imaging findings and histopathology results of core biopsy.
RESULTS
A total of 437 patients were symptomatic and 30 patients had screen detected abnormalities. All of the breast lesions who underwent ultrasound guided diagnostic procedures were sonographically visible and were classified according to BI-RADS as 3, 4 or 5.
Among 467 patients who underwent needle core biopsy for diagnosis of breast lesions 187 were reported to be BI-RADS -3, 117 were reported to be BI-RADS -4 and 163 were reported to be BI-RADS -5 [Table 1]. Out of the 467 patients biopsied, 30 were for screen detected non-palpable abnormalities and 437 were symptomatic patients.
Table 1
The number of patients in the symptomatic and screen detected cases in each category

The results were non-diagnostic in five cases, (1.07%) where the specimen was inadequate to comment upon. Two of these were in the BI-RADS -3 category and three were in the BI-RADS -4 category. These patients underwent subsequent open or excision biopsies for further evaluation.
Nearly, 72% of BI-RADS -3 lesions were fibroepithelial lesions consistent with fibroadenoma. Only three patients (1.6%) had a histopathological diagnosis of carcinoma in BI-RADS -3 category lesions. In the BI-RADS -4 category lesions, 36% had fibroadenoma and 29.9% had a malignant lesion. 93.2% were malignant in the BI-RADS -5 category lesions. Three patients (1.8%) had a fibroadenoma diagnosed on pathology in the BI-RADS -5 category. All patients with a diagnosis of malignancy (190/467) underwent staging work-up and were recommended appropriate therapy [Table 2]. Among patients diagnosed with malignancy, there was one patient with solid type palpable ductal carcinoma in situ (DCIS), three tubular cancers, one lobular cancer, two mucinous cancers and one medullary cancer. The remaining were all invasive ductal cancer not otherwise specified.
Table 2
Pathology and BIRADS correlation
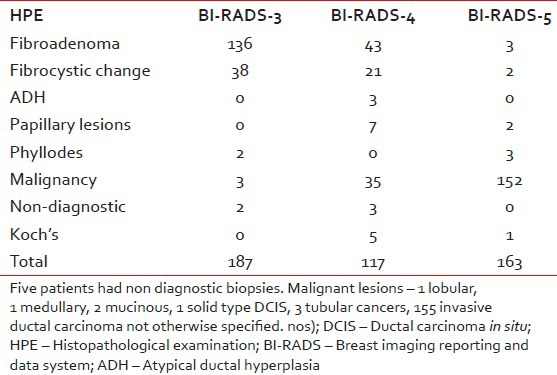
All non-malignant lesions (fibroadenoma, fibrocystic change, papillary lesions, phyllodes, atypical ductal hyperplasia) in the BI-RADS -4 and 5 categories were offered surgery. Among the total of 277 patients with a benign diagnosis 91 patients were recommended surgical excision based on the radiological and histopathological discordance. Five patients with non-diagnostic biopsies were also offered excision.
Of the 91 patients who were offered surgery, eight were lost to follow-up. Surgery was carried out in 83 patients. One patient in the BI-RADS -4 category, with a benign diagnosis of fibroepithelial lesion on core biopsy had a medullary carcinoma diagnosed after surgical excision. Papillary lesions were a diagnostic challenge even on a core needle biopsy. Out of the seven benign papillary lesions diagnosed on core biopsy in the BI-RADS category four lesions on ultrasound, three had encysted papillary carcinoma on excision. It was difficult to confirm malignancy with papillary lesions. Immunohistochemistry was used to confirm the diagnosis of carcinoma in these patients. All others had concordance between the core biopsy pathology and the post-surgical pathology result.
Complications
Two patients had a significant hematoma following the procedure. There was no core biopsy related infections or pneumothorax in our study. The core track was always excised if surgery was performed and there was no core track seedling reported in any of our excisions.
We were able to defer surgery clearly in 166/277 (60%) of patients with a clear radiological and pathological benign diagnosis. The positive predictive value (PPV) for BI-RADS -5 lesions for malignancy is 93.25%. The negative predictive value (NPV) of BI-RADS -3 lesions for cancer is 98.4%.
False negative diagnosis on core biopsy in this study was 4/467 (0.85%). There was no false positive core biopsy in our study.
Statistical analysis
The contingency table analysis (χ2 test) shows that there is a highly statistically significant association between assessments made on the mammograms and ultrasound of the breast and the core biopsy results (P - 0.000000008) [Table 3].
Table 3
Histopathological correlation with BIRADS category

The PPV for BI-RADS -5 lesions for malignancy is 93.25%. The NPV of BI-RADS -3 for cancers is 98.4%.
DISCUSSION
The reasons why FNAC is preferred over core needle biopsy in many places in the developing countries is because of the perceived less cost of the procedure and the relative ease with, which procedure is carried out.[2,3,4,5,6,7] It also does not require histological processing and therefore results are available much more quickly. In our country, where there are limited resources in many institutions, FNAC continues to be the most common method of choice for evaluating breast lesions.[8] However, published reports from Helsinki University Hospital indicate that the inadequate smear rate and inconclusive report rates are high with FNAC's resulting in an increased number of repeat biopsy procedures or surgical excision biopsy. When repeat biopsies are done, it not only increases the patient's anxiety levels, it increases the cost of the procedure and there is a time delay in arriving at a diagnosis. Resorting to a surgical excision biopsy also increases the cost and needs hospitalization/general anesthetic to do the procedure and may require definitive surgery again if it is malignant.[1] However, FNAC is still very useful in diagnosis of the chest wall recurrences and axillary node assessment prior to selection of patients for sentinel lymph node biopsy.[9]
When a malignancy is diagnosed based on a FNAC, it is difficult to categorize further into whether it is DCIS or an invasive malignancy. Sometimes, it is difficult to differentiate a lobular from ducal carcinoma. It is also not easy to do receptor (Estrogen receptor/Progesterone receptor (ER/PR) and Her 2 neu) studies with FNAC smears. In the neoadjuvant setting, the tumor might completely disappear after chemotherapy and it will not be possible to do other predictive or prognostic studies. Papillary lesions are more confidently diagnosed on core needle biopsy with immunohistochemistry if required, than by FNAC.[10] We found papillary lesions challenging even on core biopsy. Three out of seven papillary lesions on histopathology were subsequently found to be encysted papillary carcinoma.
Core biopsy is technically slightly more challenging, costs slightly more and takes a little longer to get the results.[11] Histological sections are more readily interpreted by pathologists leading to a more definite diagnosis. The PPV for BI-RADS -5 lesions for malignancy is 93.25%. The NPV of BI-RADS -3 for cancers is 98.4%. False negative rates in our study are 0.8%.
The advantages of core biopsy are as follows:[12,13,14,15]
- A good representative core biopsy is usually adequate to give a definitive diagnosis. Repeat biopsies and further excision biopsies for diagnosis can be avoided. We had five patients (1.07%) who had a non-diagnostic core biopsy, requiring further excision biopsy for diagnosis.
- A large majority of benign lesions can be followed-up without the need for surgical excision with a benign pathology report on core biopsy. We were able to defer excisions for 60% of patients with a clear rad path concordance for benign lesions.
- If the lesion is malignant it is possible to differentiate invasive from in situ malignancy and lobular from ductal carcinoma on a core biopsy.
- It is possible to do ER and PR studies and Her2 neu studies on core biopsy samples for proper pre-operative treatment planning.
- In a neoadjuvant setting core biopsy tissue might be the only tissue available for prognostic marker and predictive marker studies, as the tumor might respond completely with chemotherapy.
- Tumor banking, particularly in the neoadjuvant setting is usual for molecular studies.
The availability of automated guns has made the procedure extremely easy with image guidance. There is no role for routine testing of coagulation parameters before a core biopsy unless patient is on warfarin. Patients on aspirin or clopidogrel may bruise a bit more than usual. Patients should be warned about it, but we have not had to withhold aspirin or clopidogrel for a needle core biopsy. There is no role for routine antibiotics post-procedure. The possible risks after core biopsy include.[13]
Hematoma
US guidance with color Doppler can usually identify vasculature and this can be avoided. However, if bleeding occurs it usually stops with pressure post-procedure for 5 min. Ice compresses can be used later by patient for comfort. The presence of a large hematoma can alter the tactile discrimination for the operating surgeon at the time of surgery to ascertain the gross surgical margins in breast conservation surgery. Two of our patients had a significant hematoma following core biopsy. This however, did not affect clinical management as both patients underwent a mastectomy.
Pneumothorax
This can be avoided by the needle staying parallel to the chest wall. It must be remembered that the automated gun has a needle trajectory (throw) of about 2 cm. With some automated guns, this throw can be reduced to 1.5 cm. It is important to keep the entire length of the needle in the breast in vision without angulation toward the chest wall and staying parallel to the chest wall to avoid pneumothorax. When the lesion is close to the chest wall, a generous quantity of local anesthetic can be injected (5-10 ml) behind the lesion between the muscle and the lesion. This lifts the lesion away from the muscle and avoids pain and pneumothorax. None of our patients developed a pneumothorax post-procedure.
Infection
Infection following a core biopsy is rare and there no role for routine antibiotics. There was no core biopsy associated infections in our study.
Needle track seedling of tumor
Needle track seedling is a potential risk, but studies have not shown any increase in the incidence of local recurrence because of needle track seedling.[16,17] However, the site of the skin nick and the needle track can be kept well within the surgical incision, so it can be included in the excision. Targeting accurately and minimizing the number of passes will also reduce the risk of needle track seedling. Core tack seedling was not reported in this study following excision.
False negative result
It is possible to miss the lesion due to technical difficulties. Deep seated lesions, poor needle or lesion visualization or a dense or fibrotic breast tissue may make the procedure difficult and therefore have a discordant result.[18] When there is histological and radiologic discordance (i.e.,) when mammogram or ultrasound is suspicious of malignancy, but core biopsy histopathology is benign or inconclusive, surgical excision must always be done. Accurate targeting of the lesion will minimize the chances of false negatives. Correlating radiological findings with pathology with appropriate recommendation for further biopsy/surgical excision or follow-up should be performed. We observed false negative results in 4/467 patients. Three patients had papillary lesions, which latter on excision had encysted papillary cancer. Papillary lesions are challenging to diagnose on core biopsies and sometimes require immunohistochemistry to help with the diagnosis. One patient had a medullary carcinoma, categorized as BI-RADS -4. [Figure [Figure2a2a and andb]b] and it was missed on a core biopsy. The lesion was probably missed during biopsy.
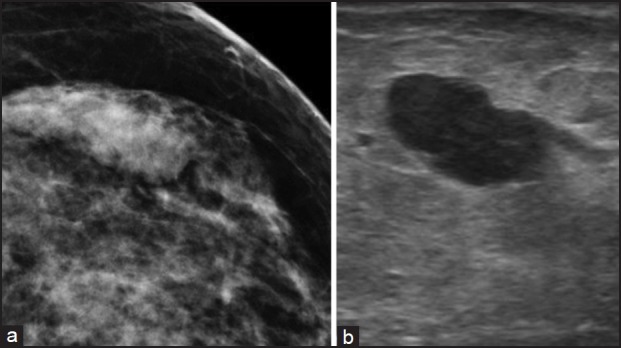
| Fig. 2 (a) Mammographic image of a medullary carcinoma, which was missed on core biopsy; (b) Ultrasound image of the medullary carcinoma which was missed on core biopsy
CONCLUSION
While in resource poor countries FNAC continues to be a valuable method in the diagnosis of palpable and non-palpable breast lesions, the practice of needle core biopsy provides the most accurate and optimal diagnostic information. Risks can be minimized with attention to detail with technique and false negatives can be minimized by having clear protocols for management after a biopsy if there is discordance in clinical, radiologic and pathological results. The PPV and NPV for cancer are high with needle core biopsy in BI-RADS3, 4 and 5 lesions. Where there is no discordance between clinical, radiology and pathology findings, surgery can be avoided in benign lesions.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES

| Fig. 1 Image showing core needle within the lesion

| Fig. 2 (a) Mammographic image of a medullary carcinoma, which was missed on core biopsy; (b) Ultrasound image of the medullary carcinoma which was missed on core biopsy


 PDF
PDF  Views
Views  Share
Share

