NEO adjuvant chemotherapy in breast cancer: What have we learned so far?
CC BY-NC-ND 4.0 · Indian J Med Paediatr Oncol 2010; 31(01): 8-17
DOI: DOI: 10.4103/0971-5851.68846
Abstract
Neoadjuvant chemotherapy (NACT) in breast cancer has undergone continuous evolution over the last few decades to establish its role in the combined modality management of these tumors. The process of evolution is still far from over. Many questions are still lurking in the minds of oncologists treating breast cancer. This review analyzes the evidence from metaanlyses, major multiinstitutional prospective trials, retrospective institutional series and systematic reviews in breast cancer to determine the current standards and controversies in NACT. The most effective drugs, their advantages, issues and controversies in delivery as well as the criteria for response are reviewed. A summary of evidence-based consensus is presented and unresolved aspects are discussed.
Publication History
Article published online:
19 November 2021
© 2010. Indian Society of Medical and Paediatric Oncology. This is an open access article published by Thieme under the terms of the Creative Commons Attribution-NonDerivative-NonCommercial-License, permitting copying and reproduction so long as the original work is given appropriate credit. Contents may not be used for commercial purposes, or adapted, remixed, transformed or built upon. (https://creativecommons.org/licenses/by-nc-nd/4.0/.)
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India
Abstract
Neoadjuvant chemotherapy (NACT) in breast cancer has undergone continuous evolution over the last few decades to establish its role in the combined modality management of these tumors. The process of evolution is still far from over. Many questions are still lurking in the minds of oncologists treating breast cancer. This review analyzes the evidence from metaanlyses, major multiinstitutional prospective trials, retrospective institutional series and systematic reviews in breast cancer to determine the current standards and controversies in NACT. The most effective drugs, their advantages, issues and controversies in delivery as well as the criteria for response are reviewed. A summary of evidence-based consensus is presented and unresolved aspects are discussed.
THE BIRTH OF NEOADJUVANT CHEMOTHERAPY (NACT): FROM HALSTEAD TO FISHER
The changing trends in management of locally advanced breast cancer actually reflect the paradigm shift in the understanding of the biology of the disease.
The Halsteadian concept of breast cancer, to begin with, as a localized disease prevailed at the end of the nineteenth century, the scene being dominated by the surgeons and the different radical surgical approaches with a hope of increasing survival.[1,2] However, contrary to their expectations, the 5-year overall survival continued to be 15–20%. A retrospective analysis of multiple case series concluded that the probability of cure was inversely proportional to initial stage of malignancy (i.e., T and N) without being influenced by the extent of radicality of thesurgery.[3–7] Studying the patterns of FAILURE lead to a better understanding of the biology of the disease and thus a multimodal approach came into vogue.
Preclinical studies being performed at the same time led to the recognition that metastatic deposits are established in patients months or years before diagnosis.[8,9]
The Fischer’s hypothesis that the disease was systemic from the very beginning ignited a holy grail search of cytotoxic agents. In various animal models, they demonstrated that removal of the primary tumor resulted in an increase in the labeling index in residual tumor cells and an increase in circulating growth-stimulating factors.[10] Administration of NACT and endocrine treatment to these animals impaired the increase in cell growth observed in residual tumor cells in untreated animals.
Introduced in the early 1970s as part of an integrated therapeutic approach to treat inoperable locally advanced breast cancer, primary, anterior, induction or NACT resulted in high responses and sufficient down-staging to allow mastectomy in some patients. The small number of pathological complete responders, which was contrary to expectations, is now the prime focus of NACT trials.
Gradually, the idea of preoperative chemotherapy was extended to include patients with large but operable early-stage breast cancer. This approach allows the tumor to be used as a measure of treatment response in vivo. More recently, the possibility has opened up for NACT to provide information on the use of clinical, pathological and molecular endpoints, which can be used as surrogate markers to predict the long-term outcome in the adjuvant setting.
Perhaps the most dramatic conceptual change in the approach to breast cancer treatment is the realization that breast cancer is a conglomerate of several molecularly defined syndromes, with distinct prognoses, clinical courses and sensitivity profiles to existing therapeutics. The anatomical accessibility of the breast provides the potential for serial biopsies to investigate molecular changes during treatment.
ADVANTAGES AND DISADVANTAGES OF NACT
Theoretically, they can be summed up as follows:[11]
| Advantages | Disadvantages |
|---|---|
| Reduction in tumor volume | Clinical/radiological staging imprecise |
| Tumor down-staging | Overtreatment of small favorable tumors |
| In vivo assessment of tumor response | Extent of surgery not confirmed |
| Less-extensive surgical resection | Loss of prognostic significance of axillary nodal status |
| Postsurgical growth spurt abrogated | Unknown relevance of surgical margins |
| Earlier introduction of a systemic therapy | Large number of drugresistant cells present |
| Response to chemotherapy serves as a marker for long-term outcome | Delays effective local therapy |
| Multiple sequential sampling of primary tumor allows evaluation of biologic changes during chemotherapy | Response of primary tumor may not correlate with response of micrometastases |
Let us review the literature for searching what level of evidence we have for these.
DOES NACT IMPROVE OVERALL SURVIVAL?
Mieog et al. conducted a systematic review[12] including 10 studies with 4,620 randomized women and 1,139 estimated deaths [Table 1]. The authors concluded that there was no survival difference between NACT and adjuvant chemotherapy [HR 0 • 98 (95%-confidence interval {c.i.} 0 • 87—1 • 09)].
Table 1
<!--caption a7-->Impact of NACT on overall survival
| Study | Overall survival rate | Weight | Hazard ratio | |
|---|---|---|---|---|
| Neoadjuvant | Adjuvant | |||
| Neoadjuvant | ||||
| Danforth[13] | 3 of 26 | 6 of 27 | 0.37 | 0.18 |
| Broet[14] | 55 of 200 | 60 of 190 | 9.55 | 0.79 |
| Mauriac[15] | 48 of 134 | 51 of 139 | 7.64 | 0.99 |
| Woolmark[16] | 221 of 742 | 218 of 751 | 40.20 | 1.02 |
| Gianni[17] | 32 of 451 | 30 of 451 | 5.39 | 1.06 |
| Van der Hage[18] | 111 of 350 | 104 of 348 | 18.57 | 1.09 |
| Subtotal | 470 of 1,903 | 469 of 1,905 | 31.73 | 1.00 |
| Test of heterogeneity | X2=5.16; | |||
| P=0.40 | ||||
| Test for overall effect | Z=0.06; | |||
| P=0.95 | ||||
| Sandwich | ||||
| Cleator[19] | 43 of 144 | 53 of 142 | 12.36 | 0.81 |
| Semiglazov[20] | 20 of 137 | 30 of 134 | 2.61 | 0.88 |
| Gazet[21] | 27 of 100 | 21 of 110 | 2.05 | 1.21 |
| Enomoto[22] | 3 of 20 | 3 of 25 | 0.45 | 1.61 |
| Subtotal | 93 of 401 | 107 of 411 | 18.47 | 0.89 |
| Test of heterogeneity | X2=1.52; | |||
| P=0.68 | ||||
| Test for overall effect | Z=0.87; | |||
| P=0.39 | ||||
| Total | 563 of 2,304 | 576 of 2,316 | 100 | 0.98 |
| Test of heterogeneity | X2=7.26; | |||
| P=0.61 | ||||
| Test for overall effect | Z=0.43; | |||
| P=0.67 | ||||
TIME TO LOCOREGIONAL RECURRENCE
Eleven studies [Table 2] reported time to locoregional recurrence data on 5,041 randomized women and 570 estimated recurrences. There was a significant difference in favor of adjuvant chemotherapy Table 1. However, in three studies, more than one-third of the patients received exclusive radiotherapy and no surgery after complete tumor regression.[13,17,18] Because of inadequate locoregional treatment after excluding these three studies, the remaining eight studies demonstrated no difference in the locoregional recurrence rate between the neoadjuvant and adjuvant groups [HR 1 • 12 (95%-c.i. 0 • 92—1 • 37)].
Table 2
<!--caption a7-->Impact of NACT on locoregional recurrence
| Study | Overall survival rate
|
Weight | Hazard ratio | |
|---|---|---|---|---|
| Neoadjuvant | Adjuvant | |||
| Ostapenko[23] | 1 of 50 | 3 of 50 | 0.72 | 0.38 |
| Gianni[17] | 8 of 438 | 22 of 875 | 5.43 | 0.75 |
| Enomoto[22] | 2 of 20 | 3 of 25 | 0.90 | 0.93 |
| Woolmark[16] | 108 of 742 | 96 of 751 | 36.90 | 1.15 |
| Van der Hage[18] | 49 of 350 | 44 of 348 | 16.77 | 1.16 |
| Gazet[21] | 24 of 100 | 104 of 348 | 18.57 | 1.09 |
| 20 of 110 | 5.19 | 1.21 | 31.73 | 1.00 |
| Cleator[19] | 13 of 44 | 9 of 142 | 4.01 | 1.50 |
| Danforth[13] | 3 of 26 | 2 of 27 | 0.90 | 1.58 |
| Subtotal | 208 of 1,870 | 199 of 2,328 | 70.82 | 1.12 |
| Test for heterogeneity | χ2=3.22, 7 d.f; | 0.88 | ||
| P=0.86 | ||||
| Test for overall effect | Z=1.15; | |||
| P=0.25 | ||||
| Inadequate local treatment | ||||
| Broet[14] | 17 of 95 | 17 of 86 | 6.15 | 0.90 |
| Broet[14] | 49 of 200 | 37 of 190 | 15.25 | 1.31 |
| Mauriac[15] | 31 of 134 | 12 of 138 | 7.78 | 2.57 |
| Subtotal | 97 of 429 | 66 of 414 | 29.18 | 1.45 |
| Test for heterogeneity | χ2=5.67, 2 d.f; | |||
| P=0.006 | ||||
| Test for overall effect | Z=2.36; | |||
| P=0.02 | ||||
| Total | 305 of 2,299 | 265 of 2,742 | 100 | 1.21 |
| Test for heterogeneity | χ2=10.76, 10 d.f; | |||
| P=0.38 | ||||
| Test for overall effect | Z=2.24; | |||
| P=0.03 | ||||
RATE OF LOCAL TREATMENT IN THE NACT AND ADJUVANT CHEMOTHERAPY ARM
There was a statistically significant decrease in the mastectomy rate [Table 3] in favor of NACT [RR 0.71 (95%-c.i. 0.67—0.75)], representing a risk difference of 16.6% (95% c.i. 15.1—18.1) (NNT 6). Of the 1,549 assessable women, 397 (25.6% [95%-c.i. 23.5—27.8)] had their surgical treatment down-staged. In 66 women, [4.3% (95%-c.i. 3.3—5.3)], tumor progression necessitated more radical surgery than originally planned.
Table 3
<!--caption a7-->Metaanalysis of neoadjuvant chemotherapy
| Study | Overall survival rate
|
Weight | Hazard ratio | |
|---|---|---|---|---|
| Neoadjuvant | Adjuvant | |||
| Cleator[19] | 16 of 149 | 31 of 144 | 2.39 | 0.50 |
| Broet[14] | 22 of 95 | 31 of 96 | 2.47 | 0.64 |
| Woolmark[16] | 239 of 743 | 302 of 752 | 22.77 | 0.80 |
| Van der Hage[18] | 203 of 323 | 262 of 341 | 19.33 | 0.82 |
| Jakesz[24] | 71 of 214 | 85 of 209 | 6.52 | 0.82 |
| Danforth[13] | 15 of 26 | 16 of 27 | 1.19 | 0.97 |
| Broet | 73 of 200 | 66 of 190 | 5.13 | 1.05 |
| Gazet[21] | 11 of 100 | 9 of 110 | 0.65 | 1.34 |
| Subtotal | 470 of 1,903 | 469 of 1,905 | 60.46 | 0.82 |
| Test of heterogeneity | χ2=9.43; 7 d.f.; P=0.22 | 199 of 2,328 | 70.82 | 1.12 |
| Test for overall effect | Z=5.10; | 0.88 | ||
| P<0> | ||||
| Gianni[17] | 154 of 438 | 579 of 875 | 29.30 | 0.53 |
| Mauriac[15] | 74 of 134 | 136 of 136 | 10.24 | 0.55 |
| Subtotal | 228 of 572 | 715 of 1,011 | 39.54 | 0.54 |
| Test of heterogeneity | χ2=0.16, 1 d.f.; P=0.69 | 37 of 190 | 15.25 | 1.31 |
| Test for overall effect | Z=11.32; | 12 of 138 | 7.78 | 2.57 |
| P<0> | ||||
| Total | 878 of 2,422 | 1,517 of 2,870 | 100 | 0.71 |
| Test of heterogeneity | χ2=53.66, 9 d.f.; P<0> | |||
| Test for overall effect | Z=10.92; | |||
| P<0> | ||||
| Total | 305 of 2,299 | 265 of 2,742 | 100 | 1.21 |
| Test for heterogeneity | χ2=10.76, 10 d.f; P=0.38 | |||
| Test for overall effect | Z=2.24; | |||
| P=0.03 | ||||
RATE OF RESPONSE TO NACT
Here, we refer to two metaaanlysis performed by Davide Mauri[25] and Fredirica Cuppone.[26] The rates of complete clinical response were statistically significantly heterogeneous (ranging from 7% to 65%; P for heterogeneity of <0 href="https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2941606/table/T0005/" target="table" class="fig-table-link figpopup" rid-figpopup="T0005" rid-ob="ob-T0005" co-legend-rid="" xss=removed>Table 4]. When both complete and partial clinical responses were considered, the difference between the extremes was smaller, but the rates were still statistically significantly heterogeneous (ranging from 45% to 83%; P for heterogeneity of <0>
Table 4
<!--caption a7-->NACT and response rates
| Study | Complete clinical response (%) | Partial clinical response (%) | Pathological response (%) |
|---|---|---|---|
| Avril, Mauriac[15] | 33 | 30 | unknown |
| Semiglazov[20] | 12 | 57 | 29 |
| Scholl[27] | 13 | 32 | unknown |
| Scholl[28] | 24 | 42 | unknown |
| Broet[14] | |||
| Makris[29] | 22 | 61 | 7 |
| Woolmark[16] | 36 | 43 | 13 |
| Gazet[21] | 25 | 26 | unknown |
| Van der hage[18] | 7 | 42 | 4 |
| Danforth[13] | 65 | 12 | 20 |
Thus, the conclusion from both these metaanalyses is that overall survival or disease-free survival (DFS) is not influenced by the timing of chemotherapy (before or after surgery) but is more likely to be influenced by the chemosensitivity of the primary lesion. The only benefit that neoadjuvant systemic therapy offers is the feasibility of breast conservation not at the cost of local recurrence, as thought earlier.
However, the recent update of the pioneering NSABP-18 study by Rastogi et al,[30] shows trends in favor of preoperative chemotherapy for DFS and OS in women less than 50 years old (hazard ratio 0.85, P 0.09 for DFS; HR 0.81, P 0.06 for OS).
WHAT IS THE BEST CHEMOTHERAPEUTIC REGIMEN FOR NACT
The introduction of combination of multiple drugs was influenced from the Goldie Coldman hypothesis, according to which the risk of resistant tumor cells can be minimized by initiating a combination of non-cross-resistant drugs. In various nonrandomized and randomized trials employing primary chemotherapy, the most commonly used regimens were CMF/FAC/AC (C=Cyclophosphamide, A=Adraiamycin, F=5FU, M=Methotrexeate). Comparative trials in metastatic and adjuvant settings showed that the efficacy of anthracycline-containing regimens were highest in terms of response rates, DFS and OS.[31–33] The same was extrapolated in the neoadjuvant setting.
ROLE OF TAXANES AS NACT
Federica Cuppone et al,[26] conducted a literature-based metaanalysis of randomized clinical trials (RCTs) to “weigh” how much taxanes add to anthracyclines as primary treatment over standard chemotherapy [Table 5]. Data from seven RCTs (2,455 patients) showed that the rate of Breast Conserving Surgery (BCS) was significantly higher for patients receiving taxanes, with an absolute difference of 3.4% (P=0.012), which translates into 29 patients NNT, without significant heterogeneity. The rate of Pathological complete response (pCR) was higher for patients receiving taxanes, although this was not statistically significant.
Table 5
<!--caption a7-->Addition of taxanes to anthracyclines in NACT
| Study | Stage of disease | No. of patients | Arms | ORR | pCR (%) |
|---|---|---|---|---|---|
| Malamos[34] | Operable | 30/30 | FEC | 50 | 0 |
| ED | 81 | 28 | |||
| Aberdeen[35], | II B and III | 162/104 | CVAP | 64 | 15 |
| Smith[36] | CVAP-D | 85 | 31 | ||
| Luprosi[37] | II and III | 90/50 | FEC | 72 | 24 |
| ED | 84 | 24 | |||
| NSABP-27[38,39] | II | 1605/1605 | AC-D | 85 | 14 |
| AC | 91 | 25 | |||
| Evans[40] | II and III | 365/363 | AC | 78 | 12 |
| AT | 88 | 8 | |||
| Semiglazov[20] | III A and III B | 103/103 | FAC | 73 | 10 |
| AT | 84 | 25 | |||
| Dieras[41] | II A, II B and III A | 247/200 | AC | 66 | 10 |
| AT | 83 | 16 |
IS THERE ANY ROLE OF DOSE-DENSE NACT?
The study by Citron et al.[42] has shown significant survival benefit with dose-dense chemotherapy in the adjuvant setting Table 7. However, such data in the neoadjuvant setting are sparse and the results are controversial.
Table 7
<!--caption a7-->Dose dense NACT
| Study | No. | Arms of the study | pCR | Rates of BCT |
|---|---|---|---|---|
| AGO Untch et al[43] | 1,069 pts | Adria 150 mg/ m2 q2wkly for 3#‐>paclitaxel 250 mg/m2 q2wkly for 3# | P=0.03 | P=0.016 |
| Adria 90 mg/ m2+docetaxel 175 mg/m2 q3wkly for 4# | ||||
| GEPARDUO[44] | 931 pts | Adria 50 mg/ m2+docetaxel 75 mg/ m2 q2wkly for 4# | 14.3% | 63.4% |
| Aria 60 mg/m2 and cyclophosphamide 600 mg/m2 q3wkly for 4# → docetaxel | 10.6% | 58.1% |
CAN ANTHRACYCLINES BE AVOIDED?
Anthracyclines, one of the most effective groups of agents for the treatment of breast cancer, should only be discarded or replaced on the basis of convincing data and, thus far, evidence to do so is lacking.
- The US Oncology (USON) 9735 trial[45] compared four cycles of AC (doxorubicin 60 mg/m2) with four cycles of docetaxel (75 mg/m2) plus the same dose of cyclophosphamide (DC).[18,19] After 5.5 years of follow-up, DFS was significantly superior in patients treated with DC and after 7 years of follow-up, OS was also significantly better in the DC arm (88% vs. 84%; hazard ratio=0.73; P=0.045)[46]).
- The BCIRG 006 trial[47] compared a nonanthracycline-containing taxane-based regimen [docetaxel, trastuzumab and carboplatin (TCH)] with two anthracycline–taxane combinations in patients with HER2-positive early breast cancer, but the study was designed primarily to evaluate the addition of trastuzumab, and the nonanthracycline-containing and anthracyclines-containing regimens differed in other ways.[30–38] Data from an interim analysis indicate that DFS and OS were significantly better in both trastuzumab arms compared with AC followed by docetaxel. There was no significant difference in efficacy between the two trastuzumab-containing arms, but there were fewer cardiac events and secondary leukemias with TCH.
SHOULD ANTHRACYCLINES AND TAXANES BE USED CONCURRENTLY OR SEQUENTIALLY?
According to the reported results, a significant benefit in pCRs in favor of taxanes appears to be restricted to a sequential strategy (all of which used docetaxel) [Tables [Tables66 and and8].8]. A trend in favor of taxanes was observed in the overall population as well, but the contribution of the sequential strategy was more than evident.
Table 6
<!--caption a7-->Results: Primary end points and sensitivity analysis (fixed effect model)
| Patients (total no of pt’s) | Relative risk (95% CI) | P value | Heterogeneity | Absolute difference (%) | Number need to treat | |
|---|---|---|---|---|---|---|
| pCR | ||||||
| Overall | 2455 | 1.22 | 0.11 | 0.05 | - | - |
| concomitant | 746 | 1.04 | 0.77 | 0.06 | - | - |
| Sequential | 1709 | 1.73 | 0.013 | 0.65 | 2.4 | 41 |
| BCS | ||||||
| Overall | 2425 | 1.11 | 0.012 | 0.43 | 3.4 | 29 |
| Concomitant | 716 | 1.22 | 0.027 | 0.78 | 5.3 | 19 |
Table 8
<!--caption a7-->Should anthracyclines and taxanes be used concurrently or sequentially?
| Effect name | Citation | Year | N total | P-value | |
|---|---|---|---|---|---|
| Concomitant-pCR | Malamos[34] | 1998 | 30 | 0.27 | |
| Concomitant-pCR | Luprosi[37] | 2000 | 50 | 1.0 | |
| Concomitant-pCR | Semiglazov[20] | 2002 | 103 | 0.006 | |
| Concomitant-pCR | Dieras[41] | 2004 | 200 | 0.828 | |
| Concomitant-pCR | Evans[40] | 2005 | 363 | 0.469 | |
| Fixed | Concomitant-pCR | 746 | 0.774 | ||
| Random | Concomitant-pCR | 746 | 0.422 | ||
| Sequential-pCR | Heys[35] | 2002 | 104 | 0.063 | |
| Sequential-pCR | Bear[38] | 2006 | 1,605 | 0.075 | |
| Fixed | Sequential-pCR | 1,709 | 0.013 | ||
| Random | Sequential-pCR | 1,709 | 0.013 | ||
| Fixed | Combined | 2,455 | 0.108 | ||
| Random | Combined | 2,455 | 0.117 |
IS THERE ANY ROLE OF A NON-CROSS-RESISTANT CHEMOTHERAPY?
The Aberdeen group enrolled 162 locally advanced breast cancer patients to four cycles of CVAP (cyclophosphamide/vincristine/doxorubicin/prednisone. Of these, 66%-of the patients who had clinical response were further randomized to four cycles of the same CVAP or four cycles of 3-weekly Docetaxel. Surgery performed at the conclusion of eight cycles found that there were significantly higher pathological complete remission rates, which also translated into a statistically superior survival rate. Thus, the study demonstrated that both the responders and the nonresponders to the initial chemotherapy regimen benefited from change over to a taxane-based chemotherapy.[35,36]
The GePAR TRIO study[47] subjected 2,090 patients of previously untreated breast cancer to two cycles of TAC. Patients whose tumors did not respond were further randomized to four cycles of TAC chemotherapy or a combination of capecitabine–vinorelbine. There was no statistical difference in the sonographic response, pathological complete response and rates of breast conservation in both the arms, concluding that addition of other agents to the anthracycline–taxane regimen in a sequential manner had no significant effect.
SHOULD ALL THE CYCLES OF CHEMOTHERAPY BE DELIVERED PREOPERATIVELY?
The National Surgical Adjuvant Breast and Bowel Project Protocol B-27 randomly assigned women (N_2,411) with operable primary breast cancer to receive either four cycles of preoperative AC followed by surgery (group I) or four cycles of AC followed by four cycles of docetaxel, followed by surgery (group II), or four cycles of AC followed by surgery and then four cycles of docetaxel (group III)[38,39] [Table 9].
Table 9
<!--caption a7-->Should all the cycles of chemotherapy be delivered preoperatively?
| Preop AC alone | Taxanes combination | P-value | |
|---|---|---|---|
| cCR | 40% | 63% | <0> |
| pCR | 13% | 26% | <0> |
| % of pts with negative nodes | 50% | 58% | <0> |
Although the initial report in 2003 showed an increase in the pathological response rate when a taxane was added preoperatively,[38] the recent update by Rastogi et al. showed no impact on the OS and DFS.[30]
WHAT IS THE IDEAL NUMBER OF CYCLES OF CHEMOTHERAPY TO BE DELIVERED PREOPERATIVELY?
In the GePAR TRIO study,[37] the first phase included randomization of responders to two cycles of TAC (n=1,390) initially and then to either a further of four or six cycles of TAC. The authors found no difference in the rates of pCR (21% vs. 23.5%; P=0.27) or breast conservation (67.5% vs. 68.5%; P=0.68). However the toxicity in the arm that received eight cycles was significantly higher. Hence, we conclude that probably six cycles of an active regimen is sufficient in the neoadjuvant setting.
WHAT IS THE ROLE OF TARGETED THERAPY IN THE NEOADJUVANT SETTING?
There are three randomized studies till date in the neoadjuvant setting evaluating the role of additional trastuzumab to standard therapy [Table 10]. The M. D. Anderson study was stopped prematurely (after 42 of a planned 165 patients) because the pCR rate with trastuzumab added to paclitaxel followed by 5-fluoruracil-epirubicin-cyclophosphamide (P→FEC) chemotherapy was astriking 65% vs. 25%) with chemotherapy alone.[48]
Table 10
<!--caption a7-->Reported randomized phase III trials with neoadjuvant trastuzumab
| Reference | Number of patients | Patient population | Design | HER2 assessment | pCR rate, No H | percentage with H | (95% c.i.) p-value |
|---|---|---|---|---|---|---|---|
| Buzdar et al., 2005,[48] 2007[49] | 42 | 65% T2 40% N0/57% N1 | P → FEC vs. P+H → FEC+H | IHC 3+ or FISH+ | 26 (9–51) | 65 (43–84) | NS |
| Gianni et al., 2007[50] | 228 | 60% T4 85% N+ | AP → P → CMF vs. AP+H → P+H → CMF+H | IHC 3+ or FISH | 23 (NR) | 43 (NR) | 0.002 |
| Untch et al., 2008[52] | 453 | NA | EC → D or EC → DX or EC → D → X vs. EC → D+H or EC → DX+H or EC → D+H → X+H | NA | 20 (NR) | 41 (NR) | <0> |
C, cyclophosphamide; CI, confidence interval; D, docetaxel; E, epirubicin; F, 5-fluoruracil; FISH, fluorescence in situ hybridization; H, trastuzumab; HER2, human epidermal growth factor receptor 2; IHC, immunohistochemistry; M, methotrexate; N, nodal status; NA, not applicable; NR, not reported; NS, not significant; P, paclitaxel; pCR, pathologic complete response; T, tumor size; X, capecitabine
The larger NeOAdjuvant Herceptin (NOAH) trial reported similar findings with trastuzumab added to doxorubicin-paclitaxel followed by paclitaxel followed by cyclophosphamide-methotrexate-5-fluoruracil (AP→P→CMF) chemotherapy.[50] Both these studies administered anthracycline chemotherapy concurrently with trastuzumab and did not report a high rate of observed cardiac toxicity, contrary to the 16%-rate of clinical grade 3/4 congestive heart failure observed in the pivotal first-line metastatic trial with concurrent trastuzumab and doxorubicin cyclophosphamide (AC).[51] The GeparQuattro study evaluating epirubicin, cyclophosphamide and docetaxel with or without capecitabine and/or trastuzumab before surgery reported a similar doubling in the observed pCR rate with the addition of trastuzumab. This study initiates trastuzumab after the completion of anthracycline therapy.
Two important ongoing neoadjuvant therapy trials are exploring the role of lapatinib in the neoadjuvant settings. Results are eagerly awaited. The schema of the study is shown in Figures Figures11 and and22.
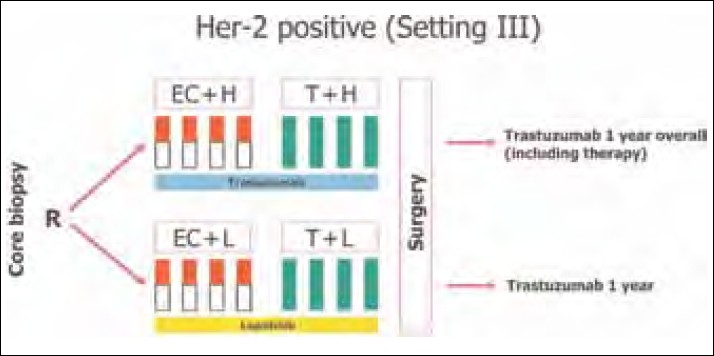
| Figure 1:GeparQuinto study schema
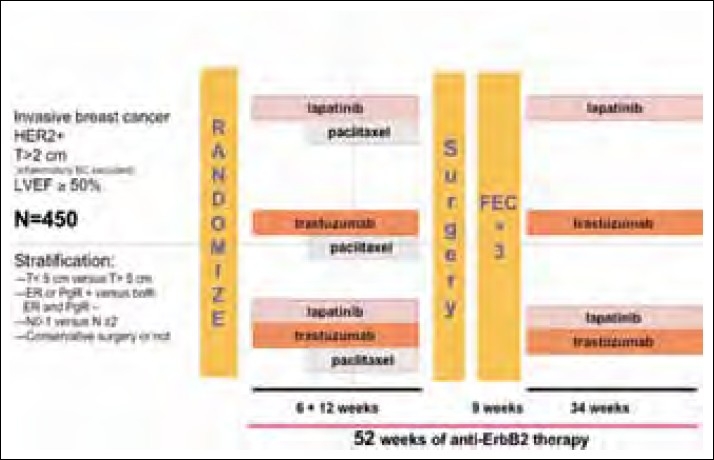
| Figure 2:Neo ALLTO study schema
GeparQuinto study (Ref Figure 1).
GeparQuinto study design for HER2-positive cohort. C, cyclophosphamide (600 mg/m2: day 1 q day 21 for four cycles); E, epirubicin (90 mg/m 2 : every 3 weeks for four cycles); H, trastuzumab (8 mg/kg: loading dose, 6 mg/kg: every 3 weeks); Her-2, human epidermal growth factor receptor 2; L, lapatinib (1,250 mg daily for 24 weeks: run-in phase cycles 1 and 5: 1,000 mg daily); R, randomization; T, docetaxel (100 mg/m2: every 3 weeks for four cycles).
DOES ADDITION OF BEVACIZUMAB HELP?
Greil et al,[53] in a phase II study, studied the efficacy and safety of the combination of Bevacizumab, docetaxel and capecitabine for her2-negative breast cancer, and found a pCR of 22%.
WHAT IS THE BEST WAY OF ASSESSMENT OF RESPONSE TO NEOADJUVANT THERAPY?
A study of 189 breast cancer patients undergoing NACT assessed tumor response to treatment with physical examination, mammography or ultrasound and compared these approaches with the gold standard, pathologic examination. The study found that false-positive rates ranged from 20% to 65%-for all modalities; false-negative rates were 10-57%.[54] The GeparTrio trial[47] revealed a sonographic complete response in 50%-of the cases examined, whereas a pathologic complete response was seen in only 5–6%-of the patients.
Advantages of magnetic resonance imaging are that it provides evidence of response as early as 6 weeks of initiation of chemotherapy. Contrast enhancement is reduced even before actual reduction in the size of the tumor. However, the foible is that the accuracy varies with the degree of response to chemotherapy and with the chemotherapeutic agent, underestimating the response in well-responding tumors and taxane-based chemotherapy.[55–63] Several studies have shown the usefulness of Positron Emission Scan in the assessment of response.[64–69] A significant decline in the standardized uptake value occurs in responders early in the course of chemotherapy.
In a study of 22 patients, after an initial course of therapy, all responding (based on Standard Uptake Value changes) tumors were identified through a decrease in SUV of >55%-below baseline (sensitivity, 100%; specificity, 85%).[68] Another study of 30 patients used PET at midtherapy assessments and reported a complete response, correlating with a 50—60%-reduction from baseline SUV.[69]
However, outside a clinical trial, these approaches are not recommended for monitoring response of breast cancer to NACT.
The gold standard for assessing response to NACT for breast cancer is still pathologic evaluation.[3] Despite the proven predictive value of pCR in this context, there is no consensus on the measurement of this important endpoint. Three of the most commonly used criteria in the literature are those of Sataloff et al.,[7] Chevallier et al.[9] and Feldman et al.[4]
A study at M.D. Anderson[72] analyzed postmastectomy pathology specimens from 241 patients treated with neoadjuvant sequential paclitaxel followed by FAC regimen and 141 patients treated with a neoadjuvant FAC regimen. The investigators then calculated the residual cancer burden (RCB), which consisted of a continuous index combining primary tumor size and cellularity as well as number and size of nodal metastases. Using multivariate analysis, they showed that RCB correlated with prognosis, independent of factors such as age, pretreatment clinical stage, hormone receptor status, hormone therapy and pathologic response (hazard ratio: 2.5; 95%-c.i. 1.7–3.69; P < 0>
NACT IN TRIPLE-NEGATIVE BREAST CANCER (TNBC)
TNBC is a heterogeneous, initially chemosensitive disease. Currently, there is no specific favored chemotherapy regimen for the treatment of TNBC. The use of taxane (paclitaxel or docetaxel) and anthracycline-based regimens, according to data for breast cancer patients in general, appear to provide higher pathological complete response rates. On the basis of the described similarities between sporadic triple-negative cancers and BRCA1-associated cancers, drugs with the ability to cause interstrand breaks, like platinum drugs, have been suggested to be used for the treatment of TNBC. This was supported by in vitro studies demonstrating the benefit of BRCA1-related tumors to these agents.[74] Because the availability of HER 2 testing is only of late, there are no studies for TNBC specifically. One study by Garber et al.[75] using preoperative single-agent cisplatin in T2/T3 TNBC reported a pCR of 23%.
A study by Carey et al.[76] evaluated responses to NACT in 107 patients with stages II and III breast cancer. Patients received neoadjuvant doxorubicin (60 mg/m2) plus cyclophosphamide (600 mg/m2) chemotherapy (AC) for four cycles, either alone or as the first component of a sequential AC-taxane neoadjuvant regimen. All patients received AC NACT at conventional doses for four cycles. Twenty-eight (26%) received AC on a dose-dense schedule (every 2 weeks), whereas the rest of the patients received AC on an every-3 weeks schedule. Most patients (80 of 107, 75%) received additional NACT following AC, which primarily involved either paclitaxel or docetaxel. PCR to chemotherapy (defined as postoperatively stage 0, no invasive cancer) was significantly better among basal-like subtype (27%), defined in this study as the immunohistochemical surrogates ER-, PR- and HER2/neu- and HER2/neu? /ER- (36%) subtypes vs. the combined luminal subtypes (7%; P=0.01). However, despite the initial chemosensitivity, patients with the basal-like and HER2/neu? /ER- subtypes had worse distant DFS (P=0.04) and OS (P=0.02) than those with the luminal subtypes This is known as the famous “Triple negative Paradox.” It has put to question all oncologists treating breast cancer who, until now, were using pCR as a surrogate for long-term survival.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- Halsted WS. I. The Results of Operations for the Cure of Cancer of the Breast Performed at the Johns Hopkins Hospital from June, 1889, to January, 1894. Ann Surg 1894;20:497-555.
- Haagensen CD, Bodian CA. A personal experience with Halsted′s radical mastectomy. Ann Surg 1984;199:143-50.
- Nemoto T, Vana J, Bedwani RN, Baker HW, McGregor FH, Murphy GP. Management and survival of female breast cancer: Results of national survey by the American College of Surgens. Cancer 1980;45:2917-24.
- Butcher HR Jr. Radical mastectomy for mammary carcinoma. Ann Sug 1963;157:165-6.
- Lacour J, Bucalossi P, Cacers E, Jacobelli G, Koszarowski T, Le M, et al. Radical mastectomy versus radical mastectomy plus internal mammary dissection. Five-year results of an international cooperative study. Cancer 1976;37:206-14.
- Schottenfeld D, Nash AG, Robbins GF, Beattie EJ Jr. Ten year results of treatment of primary operable breast carcinoma: A summary of 304 patients evaluated by the TNM system. Cancer 1976;38:1001-7.
- Robbins GF, Berg J. Curablity of patients with invasive breast carcinoma based on a 30-year study. World J Surg 1977;1:284-6.
- Shannon C, Smith I. Is there still a role for neoadjuvant therapy in breast cancer? Crit Rev Oncol Hematol 2003;45:77-90.
- Cameron DA, Anderson ED, Levack P, Hawkins RA, Anderson TJ, Leonard RC, et al. Primary systemic therapy for operable breast cancer: 10-year survival data after chemotherapy and hormone therapy. Br J Cancer 1997;76:1099-105.
- ;Bonadonna G, Bagyi GH, Valgussa P. A clinical guide to therapy. Textbook of breast cancer. 3rd ed. London and New York: Martin Dunitz; 2006.
- Bonadonna G, Valagussa P, Brambilla C, Ferrari L, Moliterni A, Terenziani M, et al. Primary chemotherapy in operable breast cancer: Eight-year experience at the Milan Cancer Institute. J Clin Oncol 1998;16:93-100.
- Fisher B, Bryant J, Wolmark N, Mamounas E, Brown A, Fisher ER, et al. Effect of preoperative chemotherapy on the outcome of women with operable breast cancer. J Clin Oncol 1998;16:2672-85.
- Mieog JS, van der Hage JA, van de Velde CJ. Neoadjuvant chemotherapy for operable breast cancer. Br J Surg 2007;94:1189-200.
- Danforth DN Jr, Cowan K, Altemus R, Merino M, Chow C, Berman A, et al. Preoperative FLAC/granulocyte-colony-stimulating factor chemotherapy for stage II breast cancer: A prospective randomized trial. Ann Surg Oncol 2003;10: 635-44.
- Broet P, Scholl SM, de la Rochefordiere A, Fourquet A, Moreau T, De Rycke Y, et al. Short and long-term effects on survival in breast cancer patients treated by primary chemotherapy: An updated analysis of a randomized trial. Breast Cancer Res Treat 1999;58:151-6.
- Mauriac L, MacGrogan G, Avril A, Durand M, Floquet A, Debled M, et al. Neoadjuvant chemotherapy for operable breast carcinoma larger than 3 cm: A unicentre randomized trial with a 124-month median follow-up. Institut Bergonie Bordeaux Group Sein (IBBGS). Ann Oncol 1999;10:47-52.
- Wolmark N, Wang J, Mamounas E, Bryant J, Fisher B. Preoperative chemotherapy in patients with operable breast cancer: Nine-year results from National Surgical Adjuvant Breast and Bowel Project B-18. J Natl Cancer Inst Monogr 2001:96-102.
- Gianni L, Baselga J, Eiermann W, Guillem Porta V, Semiglazov V, Lluch A, et al. European Cooperative Trial in Operable Breast Cancer (ECTO): Improved freedom fro progression (FFP) from adding paclitaxel (T) to doxorubicin (A) followed by cyclophosphamide methotrexate and fluorouracil (CMF). J Clin Oncol (Meeting Abstracts) 2005;23:513.
- Van der Hage JA, van de Velde CJ, Julien JP, Tubiana-Hulin M, Vandervelden C, Duchateau L. Preoperative chemotherapy in primary operable breast cancer: Results from the European Organization for Research and Treatment of Cancer trial 10902. J Clin Oncol 2001;19:4224-37.
- Cleator SJ, Makris A, Ashley SE, Lal R, Powles TJ. Good clinical response of breast cancers to neoadjuvant chemoendocrine therapy is associated with improved overall survival. Ann Oncol 2005;16:267-72.
- Semiglazov VF, Topuzov EE, Bavli JL, Moiseyenko VM, Ivanova OA, Seleznev IK, et al. Primary (neoadjuvant) chemotherapy and radiotherapy compared with primary radiotherapy alone in stage IIb-IIIa breast cancer. Ann Oncol 1994;5:591-5.
- Gazet JC, Ford HT, Gray R, McConkey C, Sutcliffe R, Quilliam J, et al. Estrogen-receptor-directed neoadjuvant therapy for breast cancer: Results of a randomised trial using formestane and methotrexate, mitozantrone and mitomycin C (MMM) chemotherapy. Ann Oncol 2001;12:685-91.
- Enomoto K, Ikeda T, Matsui A, Kitajima M, Koh J, Masamura S, et al. Neoadjuvant therapy in stage II with T>=4CM and stage III breast cancer. Eur J Cancer 1998;34:33.
- Ostapenko V, Pipiriene T, Valuckas K. Primary chemotherapy in conservative treatment of stage II breast cancer. Eur J Cancer 1998;34:34.
- Jakesz R. Comparison of pre- vs. postoperative chemotherapy in breast cancer patients: four-year results of Austrian Breast and Colorectal Cancer Study Group (ABCSG) Trial 7. J Clin Oncol (Meeting Abstracts) 2001;20:125.
- Mauri D, Pavlidis N, Ioannidis JP. Neoadjuvant versus adjuvant systemic treatment in breast cancer: A meta-analysis. J Natl Cancer Inst 2005;97:188-94.
- Cuppone F, Bria E, Carlini P, Milella M, Felici A, Sperduti I, et al. Taxanes as primary chemotherapy for early breast cancer: Meta-analysis of randomized trials. Cancer 2008; 113:238-46.
- Scholl SM, Asselain B, Palangie T, Dorval T, Jouve M, Garcia Giralt E, et al. Neoadjuvant chemotherapy in operable breast cancer. Eur J Cancer 1991;27:1668-71.
- Scholl SM, Fourquet A, Asselain B, Pierga JY, Vilcoq JR, Durand JC, et al. Neoadjuvant versus adjuvant chemotherapy in premenopausal patients with tumours considered too large for breast conserving surgery: Preliminary results of a randomised trial: S6. Eur J Cancer 1994;30A:645-52.
- Makris A, Powles TJ, Ashley SE, Chang J, Hickish T, Tidy VA, et al. A reduction in the requirements for mastectomy in a randomized trial of neoadjuvant chemoendocrine therapy in primary breast cancer. Ann Oncol 1998;9:1179-84.
- Rastogi P, Anderson SJ, Bear HD, Geyer CE, Kahlenberg MS, Robidoux A, et al. Preoperative chemotherapy: Updates of National Surgical Adjuvant Breast and Bowel Project Protocols B-18 and B-27. J Clin Oncol 2008;26:778-85.
- A′Hern RP, Smith IP, Ebbs SR. Chemotherapy and survival in advanced breast cancer: The inclusion of doxorubicin in Copper type regimens. Br J Cancer 1993;67:801-5.
- Fossati R, Confalonieri C, Torri V, Ghislandi E, Penna A, Pistotti V, et al. Cytotoxic and hormonal treatment for metastatic breast cancer: A systematic review of published randomized trials involving 31,510 women. J Clin Oncol 1998;16:3439-60.
- Polychemotherapy for early breast cancer: An overview of the randomised trials. Early Breast Cancer Trialists′ Collaborative Group. Lancet 1998;352:930-42.
- Malamos N, Kosmas C, Antonopoulos MJ. Prospective randomized study of neoadjuvant chemotherapy (NACT) with paclitaxel/epirubicin (PE) versus fluorouracil/ epirubicin/cyclophosphamide (FEC) in operable stage II-IIIA breast cancer (BC). Ann Oncol 1998;9:22.
- Heys SD, Hutcheon AW, Sarkar TK, Ogston KN, Miller ID, Payne S, et al. Neoadjuvant docetaxel in breast cancer: 3-year survival results from the Aberdeen trial. Clin Breast Cancer 2002;3:S69-74.
- Smith IC, Heys SD, Hutcheon AW, Miller ID, Payne S, Gilbert FJ, et al. Neoadjuvant chemotherapy in breast cancer: Significantly enhanced response with docetaxel. J Clin Oncol 2002;20:1456-66.
- Luporsi E, Vanlemmens L, Coudert B. Six cycles of FEC 100 vs 6 cycles of epirubicin-docetaxel (ED) as neoadjuvant chemotherapy in operable breast cancer patients (Pts): Preliminary results of a randomized phase II trial of GIREC S01. J Clin Oncol 2000;18:19.
- Bear HD, Anderson S, Brown A, Smith R, Mamounas EP, Fisher B, et al. The effect on tumor response of adding sequential preoperative docetaxel to preoperative doxorubicin and cyclophosphamide: Preliminary results from National Surgical Adjuvant Breast and Bowel Project Protocol B-27. J Clin Oncol 2003;21:4165-74.
- Bear HD, Anderson S, Smith RE, Geyer CE Jr, Mamounas EP, Fisher B, et al. Sequential preoperative or postoperative docetaxel added to preoperative doxorubicin plus cyclophosphamide for operable breast cancer: National Surgical Adjuvant Breast and Bowel Project Protocol B-27. J Clin Oncol 2006;24:2019-27.
- Evans TR, Yellowlees A, Foster E, Earl H, Cameron DA, Hutcheon AW, et al. Phase III randomized trial of doxorubicin and docetaxel versus doxorubicin and cyclophosphamide as primary medical therapy in women with breast cancer: An anglo-celtic cooperative oncology group study. J Clin Oncol 2005;23:2988-95.
- Diιras V, Fumoleau P, Romieu G, Tubiana-Hulin M, Namer M, Mauriac L, et al. Randomized parallel study of doxorubicin plus paclitaxel and doxorubicin plus cyclophosphamide as neoadjuvant treatment of patients with breast cancer. J Clin Oncol 2004;22:4958-65.
- Citron ML, Berry DA, Cirrincione C, Hudis C, Winer EP, Gradishar WJ, et al. Randomized trial of dose-dense versus conventionally scheduled and sequential versus concurrent combination chemotherapy as postoperative adjuvant treatment of node-positive primary breast cancer: First report of Intergroup Trial C9741/Cancer and Leukemia Group B Trial 9741. J Clin Oncol 2003;21:1431-9.
- Untch M, Konecy G, Ditsch N. Dose dense sequential Epirubicin-Paclitaxel as preoperative treatment of Breast cancer: Results of a randomized AGO study. Pro Am Soc Clin Onco 2002;21:133a.
- Von Minkwitz G, Raab G, Scheuette M. Dose dense versus sequential adriamycin /docetaxel combination as preoperative chemotherapy in operable breast cancer-primary endpoint analysis of GEPARDUO study. Pro Am Soc Clin Onco 2002;21:168a.
- Jones S, Holmes FA, O′Shaughnessy J, Blum JL, Vukelja SJ, McIntyre KJ, et al. Docetaxel With Cyclophosphamide Is Associated With an Overall Survival Benefit Compared With Doxorubicin and Cyclophosphamide: 7-Year Follow-Up of US Oncology Research Trial 9735. J Clin Oncol 2009;27:1177-83.
- Slamon D, Eiermann W, Robert N. Phase III randomized trial comparing doxorubicin and cyclophosphamide followed by docetaxel (AC;T) with doxorubicin and cyclophosphamide followed by docetaxel and trastuzumab (AC;TH) with docetaxel, carboplatin and trastuzumab (TCH) in HER2 positive early breast cancer patients: BCIRG 006 study. Breast Cancer Res Treat 2005;94:S5.
- Von Minckwitz G, Kόmmel S, Vogel P, Hanusch C, Eidtmann H, Hilfrich J, et al. Neoadjuvant vinorelbine-capecitabine versus docetaxel-doxorubicin-cyclophosphamide in early nonresponsive breast cancer: Phase III randomized GeparTrio trial. J Natl Cancer Inst 2008;100:542-51.
- Buzdar AU, Ibrahim NK, Francis D, Booser DJ, Thomas ES, Theriault RL, et al. Significantly higher pathologic complete remission rate after neoadjuvant therapy with trastuzumab, paclitaxel, and epirubicin chemotherapy: Results of a randomized trial in human epidermal growth factor receptor 2-positive operable breast cancer. J Clin Oncol 2005;23:3676-85.
- Buzdar AU, Valero V, Ibrahim NK, Francis D, Broglio KR, Theriault RL, et al. Neoadjuvant therapy with paclitaxel followed by 5-fluorouracil, epirubicin, and cyclophosphamide chemotherapy and concurrent trastuzumab in human epidermal growth factor receptor 2-positive operable breast cancer: An update of the initial randomized study population and data of additional patients treated with the same regimen. Clin Cancer Res 2007;13:228-33.
- Gianni L, Semiglazov V, Manikhas GM, Eiermann W, Lluch A, Tjulandin S, et al. Neoadjuvant trastuzumab in locally advanced breast cancer (NOAH): Antitumour and safety analysis. 2007 ASCO Annual Meeting Proceedings 43rd American Society of Clinical Oncology Annual Meeting; 1-5 June 2007; Chicago, IL. Abstract 532.
- Slamon DJ, Leyland-Jones B, Shak S, Fuchs H, Paton V, Bajamonde A, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med 2001;344:783-92.
- Untch M, Rezai M, Loibl S, Fasching PA, Huober J, Tesch H, et al. Neoadjuvant treatment of HER2 overexpressing primary breast cancer with trastuzumab given concomitantly to epirubicin/cyclophosphamide followed by docetaxel capecitabine. First analysis of efficacy and safety of the GBG/AGO multicenter intergroup-study ′GeparQuattro′. Presented at: 6th European Breast Cancer Conference; 15-19 April 2008; Berlin, Germany. Abstract 1LB.
- Greil R, Moik M, Reitsamer R, Ressler S, Stoll M, Namberger K, et al. Neoadjuvant bevacizumab, docetaxel and capecitabine combination therapy for HER2/neu-negative invasive breast cancer: Efficacy and safety in a phase II pilot study. Eur J Surg Oncol 2009;35:1048-54.
- ;Chagpar AB, Middleton LP, Sahin AA, Dempsey P, Buzdar AU, Mirza AN, et al. Accuracy of physical examination, ultrasonography, and mammography in predicting residual pathologic tumor size in patients treated with neoadjuvant chemotherapy. Ann Surg 2006;243:257-64.
- Partridge SC, Gibbs JE, Lu Y, Esserman LJ, Sudilovsky D, Hylton NM. Accuracy of MR imaging for revealing residual breast cancer in patients who have undergone neoadjuvant chemotherapy. AJR Am J Roentgenol 2002;179:1193-9.
- Rieber A, Brambs HJ, Gabelmann A, Heilmann V, Kreienberg R, Kόhn T. Breast MRI for monitoring response of primary breast cancer to neo-adjuvant chemotherapy. Eur Radiol 2002;12: 1711-9.
- Cheung YC, Chen SC, Su MY, See LC, Hsueh S, Chang HK, et al. Monitoring the size and response of locally advanced breast cancers to neoadjuvant chemotherapy (weekly paclitaxel and epirubicin) with serial enhanced MRI. Breast Cancer Res Treat 2003;78:51-8.
- Belli P, Romani M, Costantini M, Magistrelli A, Terribile D, Nardone L, et al. Role of magnetic resonance imaging in the pre and postchemotherapy evaluation in locally advanced breast carcinoma. Rays 2002;27:279-90.
- Bollet MA, Thibault F, Bouillon K, Meunier M, Sigal-Zafrani B, Savignoni A, et al. Role of dynamic magnetic resonance imaging in the evaluation of tumor response to preoperative concurrent radiochemotherapy for large breast cancers: A prospective phase II study. Int J Radiat Oncol Bio Phys 2007;69:13-8.
- Segara D, Krop IE, Garber JE, Winer E, Harris L, Bellon JR, et al. Does MRI predict pathologic tumor response in women with breast cancer undergoing preoperative chemotherapy? J Surg Oncol 2007;96:474-80.
- Wasser K, Sinn HP, Fink C, Klein SK, Junkermann H, Lόdemann HP, et al. Accuracy of tumor size measurement in breast cancer using MRI is influenced by histological regression induced by neoadjuvant chemotherapy. Eur Radiol 2003;13:1213-23.
- Denis F, Desbiez-Bourcier AV, Chapiron C, Arbion F, Body G, Brunereau L. Contrast enhanced magnetic resonance imaging underestimates residual disease following neoadjuvant docetaxel based chemotherapy for breast cancer. Eur J Surg Oncol 2004;30:1069-76.
- Kwong MS, Chung GG, Horvath LJ, Ward BA, Hsu AD, Carter D, et al. Postchemotherapy MRI overestimates residual disease compared with histopathology in responders to neoadjuvant therapy for locally advanced breast cancer. Cancer J 2006;12:212-21.
- Wahl RL, Zasadny K, Helvie M, Hutchins GD, Weber B, Cody R. Metabolic monitoring of breast cancer chemohormonotherapy using positron emission tomography: Initial evaluation. J Clin Oncol 1993;11:2101-11.
- Jansson T, Westlin JE, Ahlstrφm H, Lilja A, Lεngstrφm B, Bergh J. Positron emission tomography studies in patients with locally advanced and/or metastatic breast cancer: A method for early therapy evaluation? J Clin Oncol 1995;13:1470-7.
- Bassa P, Kim EE, Inoue T, Wong FC, Korkmaz M, Yang DJ, et al. Evaluation of preoperative chemotherapy using PET with fluorine-18-fluorodeoxyglucose in breast cancer. J Nucl Med 1996;37:931-8.
- Sataloff DM, Mason BA, Prestipino AJ, Seinige UL, Lieber CP, Baloch Z. J Am Coll Surg. 1995 Mar;180(3):297-306.
- Smith IC, Welch AE, Hutcheon AW, Miller ID, Payne S, Chilcott F, et al. Positron emission tomography using [(18)F]-fluorodeoxy-D-glucose to predict the pathologic response of breast cancer to primary chemotherapy. J Clin Oncol 2000;18:1676-88.
- Sataloff DM, Mason BA, Prestipino AJ, Seinige UL, Lieber CP, Baloch Z. Pathologic response to induction chemotherapy in locally advanced carcinoma of the breast: a determinant of outcome. J Am Coll Surg 1995;180:297-306.
- Chevallier B, Roche H, Olivier JP, Chollet P, Hurteloup P. Inflammatory breast cancer. Pilot study of intensive induction chemotherapy (FEC-HD) results in a high histologic response rate. Am J Clin Oncol 1993;16:223-8.
- Feldman LD, Hortobagyi GN, Buzdar AU, Ames FC, Blumenschein GR. Pathological assessment of response to induction chemotherapy in breast cancer. Cancer Res 1986;46:2578-81.
- Symmans WF, Peintinger F, Hatzis C, Rajan R, Kuerer H, Valero V, et al. Measurement of residual breast cancer burden to predict survival after neoadjuvant chemotherapy. J Clin Oncol 2007;25:4414-22.
- Bhattacharyya A, Ear US, Koller BH, Weichselbaum RR, Bishop DK. The breast cancer susceptibility gene BRCA1 is required for subnuclear assembly of Rad51 and survival following treatment with the DNA crosslinking agent cisplatin. J Biol Chem 2000;275:23899-903.
- Garber JE, Richardson A, Harris LN. Neo-adjuvant cisplatin (CDDP) in triple-negative breast cancer (BC). Proceedings San Antonio Breast Cancer Symposium, 2006.
- Carey LA, Dees EC, Sawyer L, Gatti L, Moore DT, Collichio F, et al. The triple negative paradox: primary tumor chemosensitivity of breast cancer subtypes. Clin Cancer Res 2007;13:2329-34.

| Figure 1:GeparQuinto study schema

| Figure 2:Neo ALLTO study schema
References
- Halsted WS. I. The Results of Operations for the Cure of Cancer of the Breast Performed at the Johns Hopkins Hospital from June, 1889, to January, 1894. Ann Surg 1894;20:497-555.
- Haagensen CD, Bodian CA. A personal experience with Halsted′s radical mastectomy. Ann Surg 1984;199:143-50.
- Nemoto T, Vana J, Bedwani RN, Baker HW, McGregor FH, Murphy GP. Management and survival of female breast cancer: Results of national survey by the American College of Surgens. Cancer 1980;45:2917-24.
- Butcher HR Jr. Radical mastectomy for mammary carcinoma. Ann Sug 1963;157:165-6.
- Lacour J, Bucalossi P, Cacers E, Jacobelli G, Koszarowski T, Le M, et al. Radical mastectomy versus radical mastectomy plus internal mammary dissection. Five-year results of an international cooperative study. Cancer 1976;37:206-14.
- Schottenfeld D, Nash AG, Robbins GF, Beattie EJ Jr. Ten year results of treatment of primary operable breast carcinoma: A summary of 304 patients evaluated by the TNM system. Cancer 1976;38:1001-7.
- Robbins GF, Berg J. Curablity of patients with invasive breast carcinoma based on a 30-year study. World J Surg 1977;1:284-6.
- Shannon C, Smith I. Is there still a role for neoadjuvant therapy in breast cancer? Crit Rev Oncol Hematol 2003;45:77-90.
- Cameron DA, Anderson ED, Levack P, Hawkins RA, Anderson TJ, Leonard RC, et al. Primary systemic therapy for operable breast cancer: 10-year survival data after chemotherapy and hormone therapy. Br J Cancer 1997;76:1099-105.
- ;Bonadonna G, Bagyi GH, Valgussa P. A clinical guide to therapy. Textbook of breast cancer. 3rd ed. London and New York: Martin Dunitz; 2006.
- Bonadonna G, Valagussa P, Brambilla C, Ferrari L, Moliterni A, Terenziani M, et al. Primary chemotherapy in operable breast cancer: Eight-year experience at the Milan Cancer Institute. J Clin Oncol 1998;16:93-100.
- Fisher B, Bryant J, Wolmark N, Mamounas E, Brown A, Fisher ER, et al. Effect of preoperative chemotherapy on the outcome of women with operable breast cancer. J Clin Oncol 1998;16:2672-85.
- Mieog JS, van der Hage JA, van de Velde CJ. Neoadjuvant chemotherapy for operable breast cancer. Br J Surg 2007;94:1189-200.
- Danforth DN Jr, Cowan K, Altemus R, Merino M, Chow C, Berman A, et al. Preoperative FLAC/granulocyte-colony-stimulating factor chemotherapy for stage II breast cancer: A prospective randomized trial. Ann Surg Oncol 2003;10: 635-44.
- Broet P, Scholl SM, de la Rochefordiere A, Fourquet A, Moreau T, De Rycke Y, et al. Short and long-term effects on survival in breast cancer patients treated by primary chemotherapy: An updated analysis of a randomized trial. Breast Cancer Res Treat 1999;58:151-6.
- Mauriac L, MacGrogan G, Avril A, Durand M, Floquet A, Debled M, et al. Neoadjuvant chemotherapy for operable breast carcinoma larger than 3 cm: A unicentre randomized trial with a 124-month median follow-up. Institut Bergonie Bordeaux Group Sein (IBBGS). Ann Oncol 1999;10:47-52.
- Wolmark N, Wang J, Mamounas E, Bryant J, Fisher B. Preoperative chemotherapy in patients with operable breast cancer: Nine-year results from National Surgical Adjuvant Breast and Bowel Project B-18. J Natl Cancer Inst Monogr 2001:96-102.
- Gianni L, Baselga J, Eiermann W, Guillem Porta V, Semiglazov V, Lluch A, et al. European Cooperative Trial in Operable Breast Cancer (ECTO): Improved freedom fro progression (FFP) from adding paclitaxel (T) to doxorubicin (A) followed by cyclophosphamide methotrexate and fluorouracil (CMF). J Clin Oncol (Meeting Abstracts) 2005;23:513.
- Van der Hage JA, van de Velde CJ, Julien JP, Tubiana-Hulin M, Vandervelden C, Duchateau L. Preoperative chemotherapy in primary operable breast cancer: Results from the European Organization for Research and Treatment of Cancer trial 10902. J Clin Oncol 2001;19:4224-37.
- Cleator SJ, Makris A, Ashley SE, Lal R, Powles TJ. Good clinical response of breast cancers to neoadjuvant chemoendocrine therapy is associated with improved overall survival. Ann Oncol 2005;16:267-72.
- Semiglazov VF, Topuzov EE, Bavli JL, Moiseyenko VM, Ivanova OA, Seleznev IK, et al. Primary (neoadjuvant) chemotherapy and radiotherapy compared with primary radiotherapy alone in stage IIb-IIIa breast cancer. Ann Oncol 1994;5:591-5.
- Gazet JC, Ford HT, Gray R, McConkey C, Sutcliffe R, Quilliam J, et al. Estrogen-receptor-directed neoadjuvant therapy for breast cancer: Results of a randomised trial using formestane and methotrexate, mitozantrone and mitomycin C (MMM) chemotherapy. Ann Oncol 2001;12:685-91.
- Enomoto K, Ikeda T, Matsui A, Kitajima M, Koh J, Masamura S, et al. Neoadjuvant therapy in stage II with T>=4CM and stage III breast cancer. Eur J Cancer 1998;34:33.
- Ostapenko V, Pipiriene T, Valuckas K. Primary chemotherapy in conservative treatment of stage II breast cancer. Eur J Cancer 1998;34:34.
- Jakesz R. Comparison of pre- vs. postoperative chemotherapy in breast cancer patients: four-year results of Austrian Breast and Colorectal Cancer Study Group (ABCSG) Trial 7. J Clin Oncol (Meeting Abstracts) 2001;20:125.
- Mauri D, Pavlidis N, Ioannidis JP. Neoadjuvant versus adjuvant systemic treatment in breast cancer: A meta-analysis. J Natl Cancer Inst 2005;97:188-94.
- Cuppone F, Bria E, Carlini P, Milella M, Felici A, Sperduti I, et al. Taxanes as primary chemotherapy for early breast cancer: Meta-analysis of randomized trials. Cancer 2008; 113:238-46.
- Scholl SM, Asselain B, Palangie T, Dorval T, Jouve M, Garcia Giralt E, et al. Neoadjuvant chemotherapy in operable breast cancer. Eur J Cancer 1991;27:1668-71.
- Scholl SM, Fourquet A, Asselain B, Pierga JY, Vilcoq JR, Durand JC, et al. Neoadjuvant versus adjuvant chemotherapy in premenopausal patients with tumours considered too large for breast conserving surgery: Preliminary results of a randomised trial: S6. Eur J Cancer 1994;30A:645-52.
- Makris A, Powles TJ, Ashley SE, Chang J, Hickish T, Tidy VA, et al. A reduction in the requirements for mastectomy in a randomized trial of neoadjuvant chemoendocrine therapy in primary breast cancer. Ann Oncol 1998;9:1179-84.
- Rastogi P, Anderson SJ, Bear HD, Geyer CE, Kahlenberg MS, Robidoux A, et al. Preoperative chemotherapy: Updates of National Surgical Adjuvant Breast and Bowel Project Protocols B-18 and B-27. J Clin Oncol 2008;26:778-85.
- A′Hern RP, Smith IP, Ebbs SR. Chemotherapy and survival in advanced breast cancer: The inclusion of doxorubicin in Copper type regimens. Br J Cancer 1993;67:801-5.
- Fossati R, Confalonieri C, Torri V, Ghislandi E, Penna A, Pistotti V, et al. Cytotoxic and hormonal treatment for metastatic breast cancer: A systematic review of published randomized trials involving 31,510 women. J Clin Oncol 1998;16:3439-60.
- Polychemotherapy for early breast cancer: An overview of the randomised trials. Early Breast Cancer Trialists′ Collaborative Group. Lancet 1998;352:930-42.
- Malamos N, Kosmas C, Antonopoulos MJ. Prospective randomized study of neoadjuvant chemotherapy (NACT) with paclitaxel/epirubicin (PE) versus fluorouracil/ epirubicin/cyclophosphamide (FEC) in operable stage II-IIIA breast cancer (BC). Ann Oncol 1998;9:22.
- Heys SD, Hutcheon AW, Sarkar TK, Ogston KN, Miller ID, Payne S, et al. Neoadjuvant docetaxel in breast cancer: 3-year survival results from the Aberdeen trial. Clin Breast Cancer 2002;3:S69-74.
- Smith IC, Heys SD, Hutcheon AW, Miller ID, Payne S, Gilbert FJ, et al. Neoadjuvant chemotherapy in breast cancer: Significantly enhanced response with docetaxel. J Clin Oncol 2002;20:1456-66.
- Luporsi E, Vanlemmens L, Coudert B. Six cycles of FEC 100 vs 6 cycles of epirubicin-docetaxel (ED) as neoadjuvant chemotherapy in operable breast cancer patients (Pts): Preliminary results of a randomized phase II trial of GIREC S01. J Clin Oncol 2000;18:19.
- Bear HD, Anderson S, Brown A, Smith R, Mamounas EP, Fisher B, et al. The effect on tumor response of adding sequential preoperative docetaxel to preoperative doxorubicin and cyclophosphamide: Preliminary results from National Surgical Adjuvant Breast and Bowel Project Protocol B-27. J Clin Oncol 2003;21:4165-74.
- Bear HD, Anderson S, Smith RE, Geyer CE Jr, Mamounas EP, Fisher B, et al. Sequential preoperative or postoperative docetaxel added to preoperative doxorubicin plus cyclophosphamide for operable breast cancer: National Surgical Adjuvant Breast and Bowel Project Protocol B-27. J Clin Oncol 2006;24:2019-27.
- Evans TR, Yellowlees A, Foster E, Earl H, Cameron DA, Hutcheon AW, et al. Phase III randomized trial of doxorubicin and docetaxel versus doxorubicin and cyclophosphamide as primary medical therapy in women with breast cancer: An anglo-celtic cooperative oncology group study. J Clin Oncol 2005;23:2988-95.
- Diιras V, Fumoleau P, Romieu G, Tubiana-Hulin M, Namer M, Mauriac L, et al. Randomized parallel study of doxorubicin plus paclitaxel and doxorubicin plus cyclophosphamide as neoadjuvant treatment of patients with breast cancer. J Clin Oncol 2004;22:4958-65.
- Citron ML, Berry DA, Cirrincione C, Hudis C, Winer EP, Gradishar WJ, et al. Randomized trial of dose-dense versus conventionally scheduled and sequential versus concurrent combination chemotherapy as postoperative adjuvant treatment of node-positive primary breast cancer: First report of Intergroup Trial C9741/Cancer and Leukemia Group B Trial 9741. J Clin Oncol 2003;21:1431-9.
- Untch M, Konecy G, Ditsch N. Dose dense sequential Epirubicin-Paclitaxel as preoperative treatment of Breast cancer: Results of a randomized AGO study. Pro Am Soc Clin Onco 2002;21:133a.
- Von Minkwitz G, Raab G, Scheuette M. Dose dense versus sequential adriamycin /docetaxel combination as preoperative chemotherapy in operable breast cancer-primary endpoint analysis of GEPARDUO study. Pro Am Soc Clin Onco 2002;21:168a.
- Jones S, Holmes FA, O′Shaughnessy J, Blum JL, Vukelja SJ, McIntyre KJ, et al. Docetaxel With Cyclophosphamide Is Associated With an Overall Survival Benefit Compared With Doxorubicin and Cyclophosphamide: 7-Year Follow-Up of US Oncology Research Trial 9735. J Clin Oncol 2009;27:1177-83.
- Slamon D, Eiermann W, Robert N. Phase III randomized trial comparing doxorubicin and cyclophosphamide followed by docetaxel (AC;T) with doxorubicin and cyclophosphamide followed by docetaxel and trastuzumab (AC;TH) with docetaxel, carboplatin and trastuzumab (TCH) in HER2 positive early breast cancer patients: BCIRG 006 study. Breast Cancer Res Treat 2005;94:S5.
- Von Minckwitz G, Kόmmel S, Vogel P, Hanusch C, Eidtmann H, Hilfrich J, et al. Neoadjuvant vinorelbine-capecitabine versus docetaxel-doxorubicin-cyclophosphamide in early nonresponsive breast cancer: Phase III randomized GeparTrio trial. J Natl Cancer Inst 2008;100:542-51.
- Buzdar AU, Ibrahim NK, Francis D, Booser DJ, Thomas ES, Theriault RL, et al. Significantly higher pathologic complete remission rate after neoadjuvant therapy with trastuzumab, paclitaxel, and epirubicin chemotherapy: Results of a randomized trial in human epidermal growth factor receptor 2-positive operable breast cancer. J Clin Oncol 2005;23:3676-85.
- Buzdar AU, Valero V, Ibrahim NK, Francis D, Broglio KR, Theriault RL, et al. Neoadjuvant therapy with paclitaxel followed by 5-fluorouracil, epirubicin, and cyclophosphamide chemotherapy and concurrent trastuzumab in human epidermal growth factor receptor 2-positive operable breast cancer: An update of the initial randomized study population and data of additional patients treated with the same regimen. Clin Cancer Res 2007;13:228-33.
- Gianni L, Semiglazov V, Manikhas GM, Eiermann W, Lluch A, Tjulandin S, et al. Neoadjuvant trastuzumab in locally advanced breast cancer (NOAH): Antitumour and safety analysis. 2007 ASCO Annual Meeting Proceedings 43rd American Society of Clinical Oncology Annual Meeting; 1-5 June 2007; Chicago, IL. Abstract 532.
- Slamon DJ, Leyland-Jones B, Shak S, Fuchs H, Paton V, Bajamonde A, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med 2001;344:783-92.
- Untch M, Rezai M, Loibl S, Fasching PA, Huober J, Tesch H, et al. Neoadjuvant treatment of HER2 overexpressing primary breast cancer with trastuzumab given concomitantly to epirubicin/cyclophosphamide followed by docetaxel capecitabine. First analysis of efficacy and safety of the GBG/AGO multicenter intergroup-study ′GeparQuattro′. Presented at: 6th European Breast Cancer Conference; 15-19 April 2008; Berlin, Germany. Abstract 1LB.
- Greil R, Moik M, Reitsamer R, Ressler S, Stoll M, Namberger K, et al. Neoadjuvant bevacizumab, docetaxel and capecitabine combination therapy for HER2/neu-negative invasive breast cancer: Efficacy and safety in a phase II pilot study. Eur J Surg Oncol 2009;35:1048-54.
- ;Chagpar AB, Middleton LP, Sahin AA, Dempsey P, Buzdar AU, Mirza AN, et al. Accuracy of physical examination, ultrasonography, and mammography in predicting residual pathologic tumor size in patients treated with neoadjuvant chemotherapy. Ann Surg 2006;243:257-64.
- Partridge SC, Gibbs JE, Lu Y, Esserman LJ, Sudilovsky D, Hylton NM. Accuracy of MR imaging for revealing residual breast cancer in patients who have undergone neoadjuvant chemotherapy. AJR Am J Roentgenol 2002;179:1193-9.
- Rieber A, Brambs HJ, Gabelmann A, Heilmann V, Kreienberg R, Kόhn T. Breast MRI for monitoring response of primary breast cancer to neo-adjuvant chemotherapy. Eur Radiol 2002;12: 1711-9.
- Cheung YC, Chen SC, Su MY, See LC, Hsueh S, Chang HK, et al. Monitoring the size and response of locally advanced breast cancers to neoadjuvant chemotherapy (weekly paclitaxel and epirubicin) with serial enhanced MRI. Breast Cancer Res Treat 2003;78:51-8.
- Belli P, Romani M, Costantini M, Magistrelli A, Terribile D, Nardone L, et al. Role of magnetic resonance imaging in the pre and postchemotherapy evaluation in locally advanced breast carcinoma. Rays 2002;27:279-90.
- Bollet MA, Thibault F, Bouillon K, Meunier M, Sigal-Zafrani B, Savignoni A, et al. Role of dynamic magnetic resonance imaging in the evaluation of tumor response to preoperative concurrent radiochemotherapy for large breast cancers: A prospective phase II study. Int J Radiat Oncol Bio Phys 2007;69:13-8.
- Segara D, Krop IE, Garber JE, Winer E, Harris L, Bellon JR, et al. Does MRI predict pathologic tumor response in women with breast cancer undergoing preoperative chemotherapy? J Surg Oncol 2007;96:474-80.
- Wasser K, Sinn HP, Fink C, Klein SK, Junkermann H, Lόdemann HP, et al. Accuracy of tumor size measurement in breast cancer using MRI is influenced by histological regression induced by neoadjuvant chemotherapy. Eur Radiol 2003;13:1213-23.
- Denis F, Desbiez-Bourcier AV, Chapiron C, Arbion F, Body G, Brunereau L. Contrast enhanced magnetic resonance imaging underestimates residual disease following neoadjuvant docetaxel based chemotherapy for breast cancer. Eur J Surg Oncol 2004;30:1069-76.
- Kwong MS, Chung GG, Horvath LJ, Ward BA, Hsu AD, Carter D, et al. Postchemotherapy MRI overestimates residual disease compared with histopathology in responders to neoadjuvant therapy for locally advanced breast cancer. Cancer J 2006;12:212-21.
- Wahl RL, Zasadny K, Helvie M, Hutchins GD, Weber B, Cody R. Metabolic monitoring of breast cancer chemohormonotherapy using positron emission tomography: Initial evaluation. J Clin Oncol 1993;11:2101-11.
- Jansson T, Westlin JE, Ahlstrφm H, Lilja A, Lεngstrφm B, Bergh J. Positron emission tomography studies in patients with locally advanced and/or metastatic breast cancer: A method for early therapy evaluation? J Clin Oncol 1995;13:1470-7.
- Bassa P, Kim EE, Inoue T, Wong FC, Korkmaz M, Yang DJ, et al. Evaluation of preoperative chemotherapy using PET with fluorine-18-fluorodeoxyglucose in breast cancer. J Nucl Med 1996;37:931-8.
- Sataloff DM, Mason BA, Prestipino AJ, Seinige UL, Lieber CP, Baloch Z. J Am Coll Surg. 1995 Mar;180(3):297-306.
- Smith IC, Welch AE, Hutcheon AW, Miller ID, Payne S, Chilcott F, et al. Positron emission tomography using [(18)F]-fluorodeoxy-D-glucose to predict the pathologic response of breast cancer to primary chemotherapy. J Clin Oncol 2000;18:1676-88.
- Sataloff DM, Mason BA, Prestipino AJ, Seinige UL, Lieber CP, Baloch Z. Pathologic response to induction chemotherapy in locally advanced carcinoma of the breast: a determinant of outcome. J Am Coll Surg 1995;180:297-306.
- Chevallier B, Roche H, Olivier JP, Chollet P, Hurteloup P. Inflammatory breast cancer. Pilot study of intensive induction chemotherapy (FEC-HD) results in a high histologic response rate. Am J Clin Oncol 1993;16:223-8.
- Feldman LD, Hortobagyi GN, Buzdar AU, Ames FC, Blumenschein GR. Pathological assessment of response to induction chemotherapy in breast cancer. Cancer Res 1986;46:2578-81.
- Symmans WF, Peintinger F, Hatzis C, Rajan R, Kuerer H, Valero V, et al. Measurement of residual breast cancer burden to predict survival after neoadjuvant chemotherapy. J Clin Oncol 2007;25:4414-22.
- Bhattacharyya A, Ear US, Koller BH, Weichselbaum RR, Bishop DK. The breast cancer susceptibility gene BRCA1 is required for subnuclear assembly of Rad51 and survival following treatment with the DNA crosslinking agent cisplatin. J Biol Chem 2000;275:23899-903.
- Garber JE, Richardson A, Harris LN. Neo-adjuvant cisplatin (CDDP) in triple-negative breast cancer (BC). Proceedings San Antonio Breast Cancer Symposium, 2006.
- Carey LA, Dees EC, Sawyer L, Gatti L, Moore DT, Collichio F, et al. The triple negative paradox: primary tumor chemosensitivity of breast cancer subtypes. Clin Cancer Res 2007;13:2329-34.


 PDF
PDF  Views
Views  Share
Share

