Neoadjuvant chemotherapy in advanced epithelial ovarian cancer: A survival study
CC BY-NC-ND 4.0 · Indian J Med Paediatr Oncol 2015; 36(01): 38-42
DOI: DOI: 10.4103/0971-5851.151781
Abstract
Context: Patients with advanced ovarian cancer have a poor prognosis in spite of the best possible care. Primary debulking surgery has been the standard of care in advanced ovarian cancer; however, it is associated with high mortality and morbidity rates as shown in various studies. Several studies have discussed the benefit of neoadjuvant chemotherapy in patients with advanced ovarian cancer. Aims: This study aims to evaluate the survival statistics of the patients who have been managed with interval debulking surgery (IDS) from January 2007 to December 2009. Materials and Methods: During the period from January 2007 to December 2009, a retrospective analysis of 104 patients who underwent IDS for stage IIIC or IV advanced epithelial ovarian cancer at our institute were selected for the study. IDS was attempted after three to five courses of chemotherapy with paclitaxal (175 mg/m 2 ) and carboplatin (5-6 of area under curve). Overall survival (OS) and progression free survival (PFS) were compared with results of primary debulking study from existing literature. OS and PFS rates were estimated by means of the Kaplan-Meier method. Results were statistically analyzed by IBM SPSS Statistics 19. Results: The median OS was 26 months and the median PFS was 18 months. In multivariate analysis it was found that both OS and PFS was affected by the stage, and extent of debulking. Conclusions: Neoadjuvant chemotherapy, followed by surgical cytoreduction is a promising treatment strategy for the management of advanced epithelial ovarian cancers.
Keywords
Interval debulking surgery - neoadjuvant chemotherapy - ovarian cancer - primary debulking surgeryPublication History
Article published online:
12 July 2021
© 2015. Indian Society of Medical and Paediatric Oncology. This is an open access article published by Thieme under the terms of the Creative Commons Attribution-NonDerivative-NonCommercial-License, permitting copying and reproduction so long as the original work is given appropriate credit. Contents may not be used for commercial purposes, or adapted, remixed, transformed or built upon. (https://creativecommons.org/licenses/by-nc-nd/4.0/.)
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India
Abstract
Context:
Patients with advanced ovarian cancer have a poor prognosis in spite of the best possible care. Primary debulking surgery has been the standard of care in advanced ovarian cancer; however, it is associated with high mortality and morbidity rates as shown in various studies. Several studies have discussed the benefit of neoadjuvant chemotherapy in patients with advanced ovarian cancer.
Aims:
This study aims to evaluate the survival statistics of the patients who have been managed with interval debulking surgery (IDS) from January 2007 to December 2009.
Materials and Methods:
During the period from January 2007 to December 2009, a retrospective analysis of 104 patients who underwent IDS for stage IIIC or IV advanced epithelial ovarian cancer at our institute were selected for the study. IDS was attempted after three to five courses of chemotherapy with paclitaxal (175 mg/m2 ) and carboplatin (5-6 of area under curve). Overall survival (OS) and progression free survival (PFS) were compared with results of primary debulking study from existing literature. OS and PFS rates were estimated by means of the Kaplan-Meier method. Results were statistically analyzed by IBM SPSS Statistics 19.
Results:
The median OS was 26 months and the median PFS was 18 months. In multivariate analysis it was found that both OS and PFS was affected by the stage, and extent of debulking.
Conclusions:
Neoadjuvant chemotherapy, followed by surgical cytoreduction is a promising treatment strategy for the management of advanced epithelial ovarian cancers.
INTRODUCTION
Treatment of advanced stage epithelial ovarian cancer still remains a challenge. The currently accepted management of advanced stage ovarian cancer is primary debulking surgery (PDS) followed by chemotherapy. [1,2,3,4] Many studies have shown that PDS is associated with high mortality and morbidity rates. [5,6,7,8] The morbidity is particularly higher in patients with extensive intraabdominal disease and poor general condition of the patients. It is noteworthy to mention that most patients who presents to our institution are poor, nutritionally deprived and frail and hence cannot tolerate the stress of prolonged surgery. Several studies have discussed the benefit of neoadjuvant chemotherapy (NACT) in patients with advanced ovarian cancer. [9,10,11,12,13,14] Interval debulking surgery (IDS) is the standard of care at our center for patients with advanced epithelial ovarian cancer. In the present study, we have presented the survival statistics of 104 patients who have been managed with IDS from January 2007 to December 2009.
MATERIALS AND METHODS
This was a retrospective study conducted in Dr B Borooah Cancer Institute, which caters to the entire cancer patient population of north eastern India. As reported in the Population Based Cancer Registries 2009-2011, the incidence of ovarian cancer was highest in the Kamrup urban district where this hospital is situated. [15] From January 2007 to December 2009, 104 patients who underwent IDS for stage IIIC or IV advanced ovarian epithelial cancer were selected for the study. Only those patients who had completed NACT were included for the study. Patients with poor nutritional status were routinely advised nutritional supplementation in consultation with a dietician. IDS was attempted after three to five courses of chemotherapy with paclitaxal (175 mg/m2) and carboplatin (5-6 of area under curve). Optimal cytoreduction was taken as macroscopic disease < 1 cm. Diagnosis was based on previous histopathology report of a biopsy specimen. If a biopsy specimen was not available, fine needle aspiration cytology of the ascitic fluid, metastatic disease or tru-cut biopsy of metastatic lesion was used. Tumor marker included CA 125 level. To rule out primary gastrointestinal (GI) tumors carcinoembryonic antigen (CEA), CA 125 to CEA ratio, CA 19.9, upper GI endoscopy and colonoscopy was done. Routine blood tests included complete blood count, liver function test, blood urea and serum creatinine. Radiologically contrast enhanced computerized tomography (CECT) abdomen and pelvis was used for staging advanced disease. The response to NACT was evaluated by clinical examination, serum CA 125 level, and CECT scan. Standard surgical procedure involved peritoneal washings, total abdominal hysterectomy with bilateral salphingoophorectomy, supracolic omentectomy. Formal retroperitoneal lymph node dissection was not performed in the patients and only nodal sampling of suspicious nodes was done. Cases with deposits in the porta hepatis, dense tumor deposits in the pouch of Douglas with infiltration into the rectal wall, extensive mesenteric deposits underwent suboptimal surgery. At the completion of adjuvant chemotherapy patients were again reassessed clinically, radiologically and by serum CA 125 estimation. Patients were kept on regular follow-up with history and clinical examination, CA 125 every 3 months and CECT scan every 6 months. The minimum follow-up period was 4 years. The primary end point of the study was overall survival (OS) and secondary end point was progression free survival (PFS). Survival was measured in months and estimated by means of the Kaplan-Meier method. The association between the explanatory variables (age, menopausal status, stage at presentation, grade, size of tumor, degree of cytoreduction) was assessed by cox-regression (unifunctional) and results were presented as hazard ratio and 95% confidence interval (CI). Results were considered as statistically significant when the two tailed P < 0.05. Results of survival were compared with existing survival studies. They were statistically analyzed by IBM SPSS Statistics 19 (Armonk, NY: IBM Corp.).
RESULTS
A total of 104 patients with advanced epithelial ovarian cancer who underwent IDS between January 2007 and December 2009 were followed-up till December 2013 and PFS and OS was determined. Clinical characteristics of the patients are presented in Table 1. All patients had received platinum-paclitaxel based chemotherapy. The median number of NACT cycles was 3 (range 3-5). Grade I hematological toxicity was observed in 28.84% of cases, Grade II in 5.7% of cases and Grade III in 1.92% of patients. There was no Grade IV hematological toxicity. Grade I GI toxicity was noted in 19.23% patients and Grade II in 3.8% of cases. No Grade III or IV GI toxicity was noted. Overall response to NACT was 95.19%. 19 patients had complete response (18.26%) and 80 patients (76.92%) had partial response to NACT. IDS was performed using a midline incision including: Hysterectomy (n = 102), salpingo-oophorectomy (n = 96), appendicectomy (n = 7) bowel resection anastomosis (n = 6), repair of bladder (n = 4), lymphadenectomy (pelvic: N = 7: paraaortic: N = 4 patients) [Table 2].
Table 1
Baseline demography and clinical characteristics
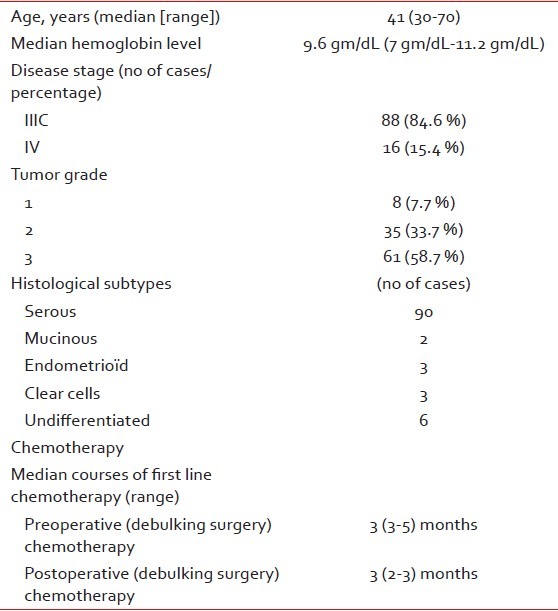
Table 2
Characteristics of debulking surgery

Postoperative complications were observed in 6 patients (5 had stitch line abscess requiring
secondary suturing and 1 developed postoperative pneumonia. All the patients recovered from these complications.
Average blood loss was 350 ml (range = 150-800 ml). Forty-three patients received blood transfusion due to
preexisting anemia and not because of blood loss. The median time period between IDS and adjuvant chemotherapy
was 31.5 days (range 21-45 days). On assessment of the survival the median OS time was 26 months [Figure 1] (CI: 24.47-27.52) and the median PFS was 18 months
(CI: 12.73-23.26) [Figure 2].
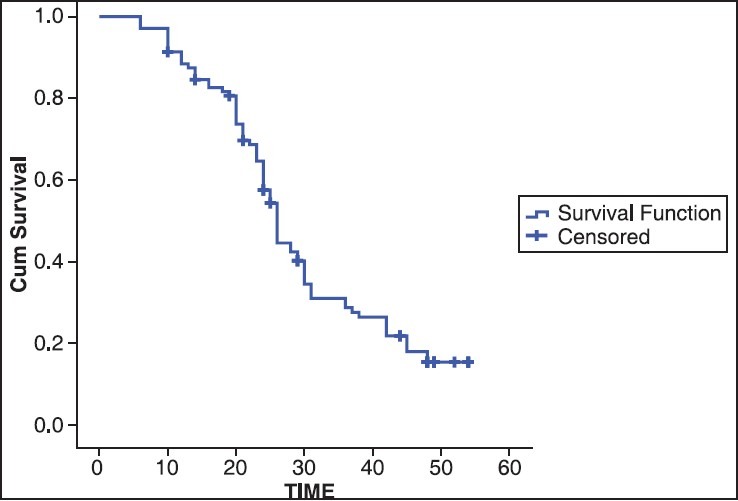
| Fig. 1 Overall survival in months
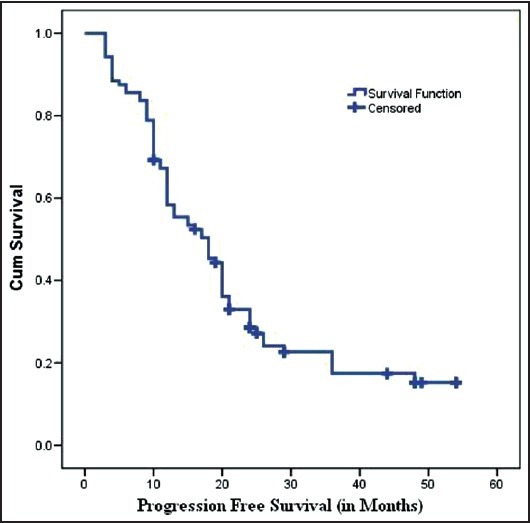
| Fig. 2 Progression free survival in months
In cox-regression analysis, it was found that both OS and PFS were affected by the stage, and
degree of debulking [Figures [Figures33 and and4].4]. The median OS for stage IIIC cancer at the time of
presentation was 26 months, whereas stage IV cancer it was 16 months (P = 0.001). The median PFS stage IIIC cancer at the time of presentation was 18 months whereas
stage IV cancer it was 4 months (P = 0.002). The OS for
patients undergoing optimal cytoreduction (<1 cm) was 26 months,
whereas it was 12 months for suboptimal cytoreduction (P = 0.001). The median PFS for optimal cytoreduction (< 1
cm) was 18 months, whereas it was 4 months for suboptimal cytoreduction (P = 0.014).
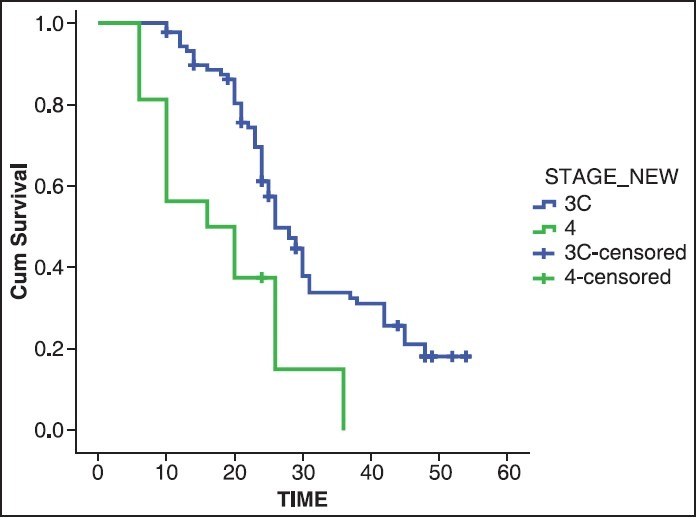
| Fig. 3 Overall survival in months (stage)
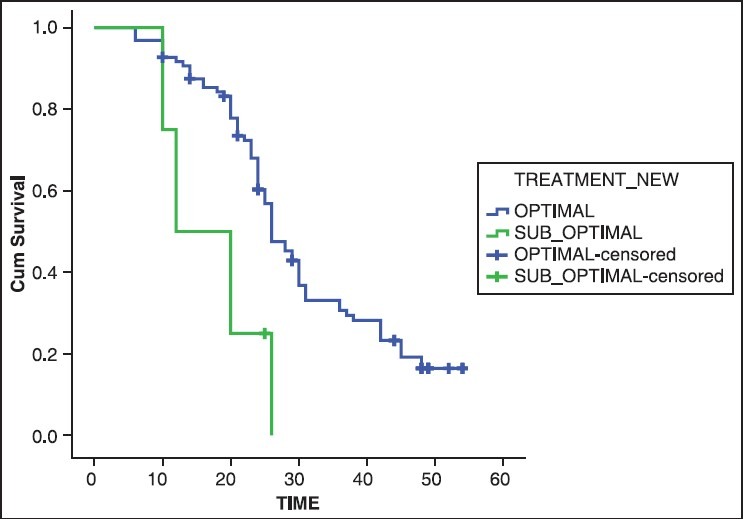
| Fig. 4 Overall survival in months (degree of debulking)
DISCUSSION
Ovarian cancer usually presents in advanced stages
with an overall 5 years survival rate of < 40% even in developed countries. [16] It continues to
be a disease of bad prognosis with high morbidity mortality. [5,6,7,8] Malnutrition is
observed in up to 67% of patients with ovarian cancer. [17] This adds up to
the high prevalence of nutritional anemia prevalent in developing countries resulting in poor
surgical performance status. [18,19] The median
hemoglobin level of our patients was 9.6 g/dL [Table 1]. Such patients therefore
cannot tolerate the stress of prolonged aggressive surgery. Even though PDS has been considered the
gold standard of advanced epithelial ovarian cancer, the serious morbidity associated with such
surgeries as reported is as high as 43%. [5,6,7,8] In addition, due
to bulky disease in advanced ovarian cancer, optimal cytoreduction is possible in < 50% of the cases. [14,16,20] NACT aims at obtaining optimum cytoreduction by
means of less aggressive surgery. It thus contributes to a decreased postoperative morbidity
and hospital stay and improves the patient's ability to tolerate postoperative adjuvant
chemotherapy. Out of 104 patients who had undergone interval debulking, optimal
cytoreduction was achieved in 92.3% of patients in our study. This was consistent with the
results of increased optimal cytoreduction rates in patients with interval debulking found
by Vergote et al. who found that
optimal cytoreduction (<1 cm) was higher (80.6%) in patients
undergoing interval cytoreduction as compared to primary surgery (41.6%). Similar
results were also found in other studies. [14,20,21] Our patients also had less intraoperative and
postoperative complications. Bowel resection and anastomosis was done in only 5.7% and
repair of bladder in 3.84%. These findings were similar to the lower incidence of
intraoperative morbidity in patients undergoing interval debulking in other studies.
[14,20] OS and PFS in our study was 26 months and 18
months respectively similar to the survival in patients undergoing interval debulking in
the study by Vergote et al.,
Morice et al. and CHORUS trials.
[14,21,22] OS
in our study was however low when it was compared to the study by Kumar et al. [20] who found an OS of 41 months in patients
undergoing IDS. On the other hand PFS was found to be slightly better (18 vs. 15
months). This might be explained due to the initial response to NACT, followed by
greater degree of optimal cytoreduction, which reflected on the PFS. On the other hand,
poor OS as compared to the PFS might be due to the fact that most of the patients did
not receive second line chemotherapy. This was either due to nonaffordability of the
second line drugs or poor performance status of these patients at the time of relapse. A
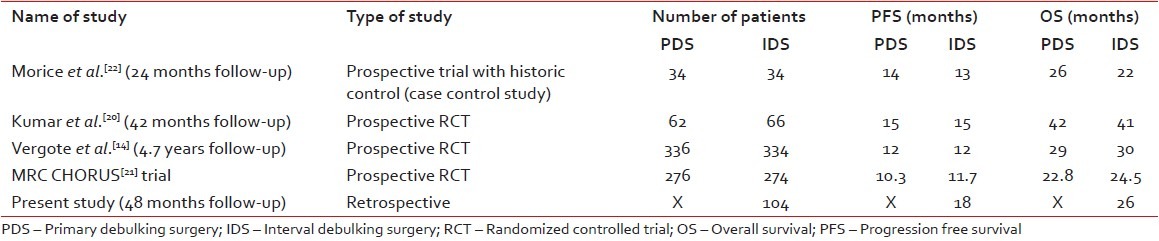
comparison between these studies is shows in Table 3. The OS and PFS
in our study was found to be affected by degree of debulking, which was also found in
other studies. [14,21,22,23,24] We
have also found that OS and PFS was significantly less in patients who were stage IV at
the time of presentation reflecting the poor long term prognosis of these patients in
spite of initial response to chemotherapy. [25]
Table 3
Comparison table
CONCLUSION
Our study has shown that NACT is a promising approach in Indian patients with advanced ovarian cancer as a significant number of patients respond to it leading to increased optimal cytoreduction. This has resulted in comparable survival for patients without increased morbidity and greater percentage of patients being able to undergo optimal cytoreduction.
REFERENCES

| Fig. 1 Overall survival in months

| Fig. 2 Progression free survival in months

| Fig. 3 Overall survival in months (stage)

| Fig. 4 Overall survival in months (degree of debulking)


 PDF
PDF  Views
Views  Share
Share

