Neoadjuvant chemotherapy in oral cancers: Selecting the right patients
CC BY-NC-ND 4.0 · Indian J Med Paediatr Oncol 2015; 36(03): 148-153
DOI: DOI: 10.4103/0971-5851.166716
Abstract
The standard of care treatment for oral squamous cell carcinoma (OSCC) at present, consist of surgical resection followed by adjuvant radiotherapy and chemotherapy as indicated. Despite recent advances the overall prognosis remains guarded. Role of neoadjuvant chemotherapy is being explored with premise of reducing extent of surgical resection, improving loco-regional control and decreasing distant metastasis, thereby improving treatment outcomes by decreasing mortality and morbidity. However, indications of neoadjuvant chemotherapy in oral cancers are not clearly defined. Majority of studies have failed to demonstrate a significant benefit of neoadjuvant chemotherapy in terms of loco regional control and overall survival in resectable OSCC. In a select subset of patients with locally very advanced and unresectable OSCC, neoadjuvant chemotherapy has been shown to cause tumor shrinkage and improve resectability. These hypothesis generating findings of reduction in distant metastasis, improved resectability and functional outcome, however need further validation. In summary, the role of neoadjuvant chemotherapy for OSCC remains investigational and has a limited role outside clinical trial.
Keywords
Neoadjuvant chemotherapy - oral squamous cell carcinoma - advanced oral cancers - unresectable oral cancersPublication History
Article published online:
12 July 2021
© 2015. Indian Society of Medical and Paediatric Oncology. This is an open access article published by Thieme under the terms of the Creative Commons Attribution-NonDerivative-NonCommercial-License, permitting copying and reproduction so long as the original work is given appropriate credit. Contents may not be used for commercial purposes, or adapted, remixed, transformed or built upon. (https://creativecommons.org/licenses/by-nc-nd/4.0/.)
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India
Abstract
The standard of care treatment for oral squamous cell carcinoma (OSCC) at present, consist of surgical resection followed by adjuvant radiotherapy and chemotherapy as indicated. Despite recent advances the overall prognosis remains guarded. Role of neoadjuvant chemotherapy is being explored with premise of reducing extent of surgical resection, improving loco-regional control and decreasing distant metastasis, thereby improving treatment outcomes by decreasing mortality and morbidity. However, indications of neoadjuvant chemotherapy in oral cancers are not clearly defined. Majority of studies have failed to demonstrate a significant benefit of neoadjuvant chemotherapy in terms of loco regional control and overall survival in resectable OSCC. In a select subset of patients with locally very advanced and unresectable OSCC, neoadjuvant chemotherapy has been shown to cause tumor shrinkage and improve resectability. These hypothesis generating findings of reduction in distant metastasis, improved resectability and functional outcome, however need further validation. In summary, the role of neoadjuvant chemotherapy for OSCC remains investigational and has a limited role outside clinical trial.
INTRODUCTION
Oral squamous cell carcinoma (OSCC) is the most common type of tumor in the oral cavity.[1] Early stage tumors account for about 30% of the tumors. The majority of the tumors are locally advanced and have relatively poor prognosis with 5 years survivals < 50-60%.[2,3,4] At present, the standard of care for resectable locally advanced OSCC is the surgical treatment of the primary tumor and neck followed by postoperative radiotherapy or chemoradiotherapy, depending on the presence of intermediate- or high-risk features.[5] The pattern of failure for oral cancer is predominantly locoregional.[6] Extensive procedures are required in these locally advanced cancers which are associated with a substantial amount of cosmetic deformity and functional morbidity. The recent advances in reconstruction techniques have enabled the possibility of wider resections with limited morbidity.[7] However, resectability of the tumor must be a fine balance between achieving negative surgical margins with acceptable functional and cosmetic deformities.[8]
Neoadjuvant chemotherapy (NACT) in head and neck cancers has been investigated for long with an aim of reducing surgical margins, distant metastasis rates, and improving outcomes. The meta-analysis of chemotherapy on head and neck cancer meta-analysis of 31 trials involving more than 5000 patients failed to demonstrate significant survival benefit following induction chemotherapy. However, the trials that used 5- fluorouracil (5-FU) and cisplatin as a part of NACT regime showed significant overall survival (OS) benefit compared to other combination regimens and single agent NACT. The trials included in the meta-analysis did not specifically address oral cavity cancers and had less number of OSCC patient.[9,10] Most of these trials were from the pretaxane era, and impact of taxanes in the neoadjuvant setting is not addressed with this meta-analysis. The interest in NACT has been rekindled by recent studies such as TAX 323 and 324 which included taxane (docetaxel) along with fluorouracil and cisplatin (TPF) containing regimen. The TPF induction regimen showed improved survival in advanced head and neck squamous cell carcinoma (HNSCC) as compared to patients receiving an induction regimen with cisplatin and fluorouracil (PF) alone. However, these trials included various head and neck subsites and also were not exclusively designed for OSCC.[11,12,13]
The aim of this paper is to critically review the current evidence for NACT in locally advanced OSCC and suggest an algorithmic approach to the patient population who might benefit from NACT for OSCC.
NEOADJUVANT CHEMOTHERAPY FOR RESECTABLE ORAL SQUAMOUS CELL CARCINOMA
Okura et al. in 1998 published a retrospective single institution review of NACT in patients with 141 operable oral cavity cancers. The NACT consisting of two cycles of cisplatin, vincristine, and peplomycin, with or without mitomycin C showed overall response rate was 51.5%, and there was a documented decrease in the rates of distant metastases. There was no decrease in overall or disease-free survival when all 141 patients were compared. The type of surgery performed, the response to chemotherapy according to the T stage, the impact of effective chemotherapy and the adequacy of surgical margins achieved were not reported.[14] The drawbacks of the trial were that this was a single institution retrospective study with a combination of drugs which were not tested rigorously in head and neck cancer.
With regards to the use of NACT prior to surgery in resectable oral cavity cancers, at least two well-structured and adequately powered randomized trials have been published. Licitra et al. in their article, published the results of a randomized, multicenter trial for 195 patients with advanced resectable, stage T2-T4 (>3 cm), N0-N2, M0 untreated squamous cell carcinoma (SCC) of the oral cavity. Patients were randomly assigned to three cycles of cisplatin and fluorouracil followed by surgery (chemotherapy arm) or surgery alone (control arm). Complete or partial clinical response of the primary tumor was observed in 28 (33%) and 42 (49%) patients, respectively, adding up to a total of 82% objective response rate. However, stage IV tumors the response rate was only 18%. Segmental mandibulectomy was performed in the chemotherapy arm (31% vs. 52% in the control group) There was no increase in the number of positive surgical margins. There was no difference in terms of locoregional recurrence, distant metastasis and OS between the two arms. However, it was observed that patients achieving pathologically complete response had statistically significant higher 5 years survival rates.[15]
Long-term results of the same trial published by Bossi et al., with a median follow-up of 11.5 years showed no difference in the incidence of locoregional relapse between chemotherapy and control group (P = 0.6337), nor in distant metastasis development (P = 0.1527), or OS (P = 0.3402). Patients with a pathological complete response (pCR) to the NACT had a higher probability of survival than those without (10-year OS: 76.2% vs. 41.3%, P = 0.0004).[16]
Zhong et al. published the results of their prospective open-label phase III trial of 256 untreated stages III or IVA locally advanced resectable OSCC. Patients were randomized into two groups who received two cycles of NACT (TPF) (docetaxel 75 mg/m2 on day 1, cisplatin 75 mg/m2 on day 1, and fluorouracil 750 mg/m2 on days 1-5) followed by radical surgery and postoperative radiotherapy (54-66 Gy) versus up-front radical surgery and postoperative radiotherapy. The primary endpoint was OS, and the secondary endpoints included local control and safety. The pathological response rate was 13.4%. A statistically insignificant lower rate of distant metastasis in the NACT arm was observed (5.5% vs. 8.7%). At a median follow-up of 30 months, there was no difference in the disease-free survival and the OS between the two arms (68.8% vs. 68.2%). However, as in the trial by Licitra et al. patients in the NACT arm with a clinical response or favorable pathologic response (< 10% viable tumor cells) had superior OS and locoregional and distant control.[17]
The long-term results of this study confirmed that at a median follow-up of 70 months there were no significant differences in survival rates between experimental and control groups. However, patients with favorable pathologic responses had improved outcomes compared to those with unfavorable pathologic responses and also to those in the control group in terms of OS, distant metastasis free survival (DMFS), local recurrence free survival which were statistically not significant. Subgroup analysis demonstrated that cN2 patients receiving TPF NACT had a better outcome than those not receiving TPF NACT, especially OS and DMFS. In addition, primary tongue carcinomas had a trend toward better OS and DMFS compared to other oral cavity subsites.[18] Although, this is a post-hoc analysis is interesting and hypothesis generating, it must be interpreted with caution.
Although neoadjuvant strategy provided no significant advantage, the chemotherapy sensitivity of oral cancers has been demonstrated in both these randomized trials. The pCR at the primary site reported by Licitra et al. is 27%. Favorable pathologic response was noted in 27.7% with pCR of 13.4% by Zhong et al. The extent of surgery after NACT was different in the trial reported by Licitra et al. with significantly lower rates of segmental mandibulectomy in the NACT arm (30.6% vs. 51.5%). While the lower rates of segmental mandibulectomy are associated with better quality of life, the impact of reduced surgical effort to achieve margin negativity in the chemotherapy group remains unclear. A meta-analysis of induction chemotherapy between 1965 and 2011 in resectable HNSCC of all subsites, which included 14 randomized control trials with 2099 patients showed a 8% reduction in rate of distant metastasis in patients receiving induction chemotherapy.[19]
In summary, multiple studies have failed to demonstrate improvement in locoregional control, OS in resectable oral cavity cancer. However, the effect of NACT on reducing distant metastasis, mandibular preservation in tumors without gross invasion of mandible and disease control in patients with higher nodal stage seems interesting and warrants further investigation.
NEOADJUVANT CHEMOTHERAPY IN LOCALLY ADVANCED CANCER
Paccagnella et al. showed in their study that patients with either stage III or IV SCC tumors of the head and neck without distant metastasis who were not candidates for surgery had a 21% OS rate at 5 years when they underwent 4 cycles of neoadjuvant cisplatin and 5-FU followed by radiotherapy as opposed to an 8% OS rate in similar patients who received radiotherapy alone. The participants in the study who were deemed resectable after treatment underwent surgical resection. These patients did not have any survival benefit with the addition of chemotherapy, however, showed significantly lower rates of distant metastasis.[20]
Hitt et al. published the first randomized trial comparing PF to PF plus paclitaxel. They included 384 patients with resectable and unresectable disease. The trial showed significant improvements in terms of response rates and time to treatment failure in favor of TPF regimen. A clear OS advantage was however observed only in unresectable disease.[21]
TAX 323 trial, compared TPF versus PF followed by radiotherapy (4 cycles) in 358 patients with locally advanced HNSCC (LAHNSCC). The primary endpoint was progression-free survival. It was observed that patients in TPF group had statistically significant PFS and OS. The proportion of oral cavity cancer patients in this study was about 17 %.[12]
TAX 324 trials, a randomized, open-label phase 3 trial consisting of 501 patients with LAHNSCC compared three cycles of TPF induction chemotherapy with three cycles of PF. Both regimens were followed by 7 weeks of chemoradiotherapy with concomitant weekly carboplatin. TPF group showed 3 years OS was 62% versus 48% in the PF group. However, oral cavity patients consisted only 13% in TPF and 15% in PF group.[11]
The long-term follow-up of 5 years of TAX 324 study showed that the OS was significantly better with TPF versus PF (hazard ratio [HR] = 0.74, 95% confidence interval [CI]: 0.58-0.94), with an estimated 5-year survival rate of 0.52 and 0.42 in the TPF and PF arms, respectively. Median survival time was 70.6 months (95% CI: 49.0-89.0 months) with TPF versus 34.8 months (the 95% CI: 22.6-48.0 months) in the PF group (P = 0.014). PFS was also significantly better with TPF (38.1 months; 95% CI: 19.3-66.1 months vs. 13.2 months, 95% CI: 10.6-20.7 months; HR = 0.75, 95% CI: 0.60-0.94). The results in the TPF group were better for laryngeal and hypopharyngeal cancers.[13]
None of these trials was designed exclusively for oral cavity cancers and only had a small proportion of patients with OSCC. The outcomes were also not compared with the outcomes with the standard of care which is surgery. Therefore, direct extrapolation to OSCC patients may not be possible. These studies clearly demonstrate the advantage of using three drugs over two drug regime for induction. However, feasibility of using TPF chemotherapy in this setting remains low in the Indian context.
NEOADJUVANT CHEMOTHERAPY IN LOCALLY VERY ADVANCED/UNRESECTABLE ORAL CAVITY CANCER
AJCC staging 2010 defines very advanced, local disease, or unresectable T4b OSCC as tumor invading the masticator space, pterygoid plates, and skull base, or encasing the internal carotid artery.[22] However, the resectability remains controversial term with limited consensus among the surgical teams. The term technically unresectable has been also been used to include tumors which are not staged as T4b but are known to carry poor prognosis and high morbidity after surgical resection. A rationale of proposing NACT in these cases is to improve the overall outcome by reducing tumor burden prior to radiation or facilitate possible resection following tumor shrinkage.
It has been seen that the results of chemoradiation or radical radiation alone in T4b cancers are not satisfactory with a disease-free survival of 1 year.[23] Primary surgical resection using compartment techniques seems to have good loco-regional control in OSCC patients with infratemporal fossa involvement. Although a small series, a study by Trivedi et al. showed good locoregional control rate using compartmental resections for tumors involving masticator space.[24] Liao et al. in a single institution study reported encouraging results on upfront surgery in T4b oral cavity cancers below the mandibular notch. The 5 years locoregional control rate was 47%.[25,26]
Patil et al. published a retrospective study of 123 patients with technically unresectable locally advanced oral cavity cancers. Unresectibility in these cases was defined as disease reaching up to the zygoma and/or soft tissue swelling up to the zygoma, extensive soft tissue involvement reaching up to the hyoid cartilage, extensive skin infiltration, and the involvement of the infratemporal fossa. The patients were given NACT with TPF or TP and assessed for resectibility. The response rate with the three drug and two drug regimens was 32.00% and 27.37%, respectively. Resectability was achieved in 17 patients with 3 drug regimen (68.00%) and 36 patients with 2 drug regimen (37.89%). The estimated median OS was 12.7 months. The estimated median survival was not reached for patients undergoing postchemotherapy resection. This was statistically significant compared to patients treated with nonsurgical modalities postchemotherapy. The estimated median OS in these patients was 8 months (P = 0.0001). They demonstrated the effectiveness of NACT in down-staging tumors and enabling radical surgery with comparable 2 years survival to primary surgery.[27]
In a subsequent article by the same group, Joshi et al. reported on the role of NACT followed by resection in T4b tumors. They assessed the efficacy and impact of NACT in T4b tumors in a retrospective study of 110 patients. 92.7% of their patients had involvement of the masticator space. 20% of patients received 3 drug regimens while the rest received various combinations of 2 drug regimen. Partial response was achieved in 28 patients, stable disease in 49 patients and progression was noted in 23 patients. Resectability was achieved in 34 (30.9%) of 110 patients. The estimated median OS in patients who underwent surgery was 18.0 months (95% CI: 13.6-22.46 months) and for those treated with nonsurgical treatment was 6.5 months (95% CI: 5.6-7.4 months) (P = 0.0001).[28] It is noteworthy to know that none of the patients had a complete response to NACT. However, resectability was achieved in 30.9% of the tumors considered unresectable prior to chemotherapy thereby improving the outcome.
In a follow-up article by Patil et al., retrospectively analyzed 721 patients with T4a and T4b OSCC deemed as technically unresectable who received NACT. 43% of these patients had sufficient reduction in tumor size that made them resectable. 3 drug regimen achieved resectability in 66.21% and two drug regimen in 40.34%. The locoregional control rate was 20.6% for the overall cohort. For patients undergoing surgery, the LRC was 32% and 15% for the nonsurgical group. The median estimated survival was 19.6 and 8.16 months, respectively.[29] Thus, NACT failed to demonstrate a significant change in improving resectability and outcome. In addition, the pattern of pathological shrinkage in advanced OSCC following NACT has not been well documented. The pathological and radiological studies in breast cancer and other cancers have demonstrated a variable pattern of tumor shrinkage following NACT. The tumors may not shrink concentrically and may have a satellite residual tumor beyond the visible tumor.[30,31] The clinical and radiological findings in advanced OSCC suggest a similar variable tumor shrinkage pattern [Figures [Figures11 and and2].2]. Further studies are warranted to clearly establish the pathological pattern of tumor shrinkage following NACT. This will help to define the extent of resection following chemotherapy.
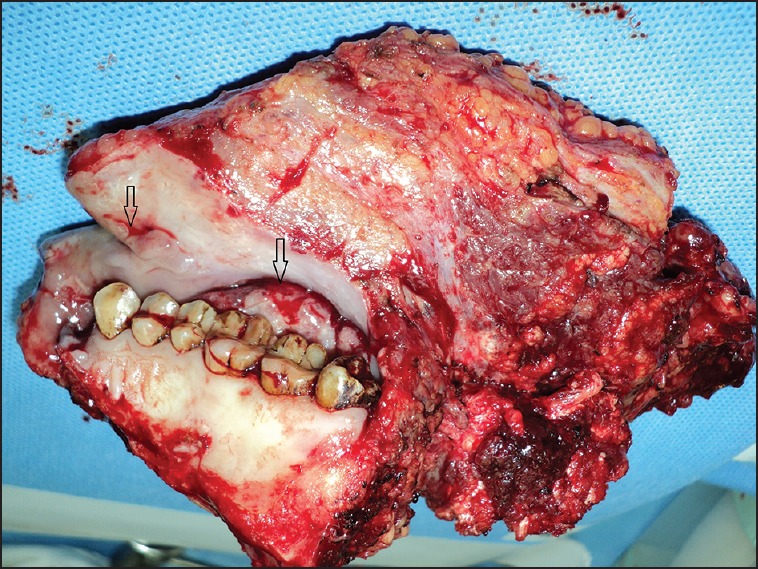
| Fig. 1 Postneoadjuvant chemotherapy clinical specimen showing nonconcentric tumor shrinkage in separate areas with intervening tissue
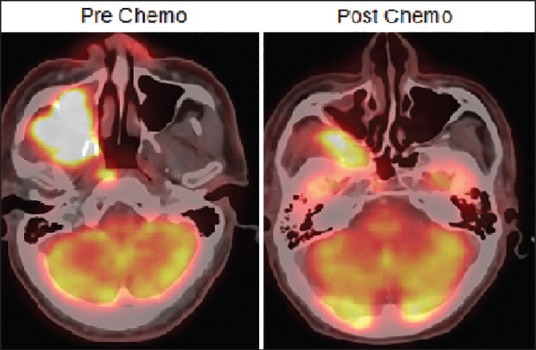
| Fig. 2 Pre- and post-neoadjuvant chemotherapy positron emission tomography-computed tomography scan showing nonconcentric shrinkage of tumor
POTENTIAL PREDICTIVE BIOMARKERS FOR NEOADJUVANT CHEMOTHERAPY IN ORAL CANCERS
As discussed earlier a significant proportion of patients with OSCC exhibit response to NACT. These patients have been shown to have better locoregional control, disease-free survival, and OS. In addition, unresectable oral cavity cancers may be considered resectable after NACT. The identification of tumor response predictors may allow a more rational selection of therapeutic strategies, sparing unnecessary toxicities to patients who would not benefit from NACT. It could further lead to the development of new drug regimens to overcome primary resistance to NACT. Multiple studies have proposed biomarkers for predicting response to chemotherapy.
Tsuji et al. retrospectively studied 70 patients with OSCC for anti-tumor effects of cisplatin-based NACT in relation to biological markers of tumor cell proliferation activity: Tumor grade, cellular DNA content, mitotic index, apoptotic index, Ki-67 positive rate, and p53 and Bax expression. It was observed that tumor grade, Bax expression, apoptotic index, and cellular DNA content correlated significantly with the anti-tumor effects of NACT in univariate analysis. By using multiple logistic analyses, tumor grade, Bax expression, and apoptotic index were selected as independent predictive factors. Using the regression equation from these results, the prediction rate for anti-tumor effects was 70%.[32]
Perrone et al. analyzed 53 pretreatment biopsies of oral cavity SCC patients receiving primary cisplatin and fluorouracil chemotherapy followed by surgery for the predictive value of TP53 mutations and their functional status on the basis of the transactivation activity of p53 mutant proteins. 28% patients achieved a pathologic complete remission (pCR) at both T and N sites, and 38 patients had residual tumor cells. Among 53 pretreatment biopsies, 24 (45%) displayed TP53 mutations: 22 single-nucleotide substitutions and two deletions. TP53 mutation predicted pCR in 4 (17%) of 24 patients and a nonfunctional mutation in only 2 (9%) of 22 patients. They concluded that the loss of function (transactivation activities) of p53 mutant proteins might predict a significant low pCR rate and a suboptimal response to cisplatin-based NACT in patients with OSCC.[33]
Yanamoto et al. retrospectively analyzed 89 patients who underwent radical surgery for tongue cancer and examined the effect of NACT on tumor local recurrence. Cancer stem cell marker (CD44v6 and ABCG2) expression was detected by immunohistochemistry. The local recurrence rate was 12.4%. Expression of CD44v6 and ABCG2 was significantly associated with regional lymph node metastasis, the pattern of invasion, depth of invasion, perineural invasion, and local recurrence, respectively. CD44v6 or ABCG2 positivity in NACT treated patients was significantly associated with local recurrence. They suggested that local recurrence in NACT treated cases was associated with cancer stem-like cells and NACT may lead to the selection and/or residue of more aggressive cancer stem-like cells.[34] There is an unmet need to develop a biomarker panel specific for predicting response to NACT.
SUMMARY
Current evidence suggests that there is a limited role for NACT in locally advanced resectable oral cancer and should not be used outside of a well-designed clinical trial. The addition of NACT has failed to demonstrate an improvement in local control rates and OS in resectable OSCC. The improvement in the distant metastasis rate among a select group of patients requires further validation. There is a need to standardize the definition of resectable advanced OSCC for both clinical practices and for inclusion in future clinical trials. Patients with unresectable oral cavity cancers may have responses to the tune of 70% with aggressive three-drug regime (TPF), and a third of these patients may benefit for subsequent surgical treatment. The patient with good response and subsequent resections has a better outcome. In view of limited benefit with NACT, newer strategies need to be conceived in order to improved outcome in patients with advanced OSCC.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES

| Fig. 1 Postneoadjuvant chemotherapy clinical specimen showing nonconcentric tumor shrinkage in separate areas with intervening tissue

| Fig. 2 Pre- and post-neoadjuvant chemotherapy positron emission tomography-computed tomography scan showing nonconcentric shrinkage of tumor


 PDF
PDF  Views
Views  Share
Share

