Neoadjuvant chemotherapy in technically unresectable carcinoma of external auditory canal
CC BY-NC-ND 4.0 · Indian J Med Paediatr Oncol 2015; 36(03): 172-175
DOI: DOI: 10.4103/0971-5851.166734
Abstract
Background: Carcinoma of external auditory canal (EAC) is a very rare malignancy with surgical resection as the main modality of treatment. The outcomes with nonsurgical modalities are very dismal. We present a retrospective analysis of 4 patients evaluating the role of neoadjuvant chemotherapy in technically unresectable cancers. Materials and Methods: This is a retrospective analysis of 4 patients from our institute from 2010 to 2014 with carcinoma EAC who were deemed unfit for surgery due to extensive disease involving occipital bone with soft tissue infiltration (n = 2), temporal dura (n = 1), left temporal lobe, and extensive soft tissue involvement (n = 1). All these patients received neoadjuvant chemotherapy with docetaxel, cisplatin and 5 fluorouracil (n = 3) and paclitaxel and cisplatin (n = 1). Results: Response evaluation showed a partial response (PR) in 3 and stable disease (SD) in 1 patient by Response Evaluation Criteria in Solid Tumors criteria. All 3 patients who received 3 drug chemotherapy had PR while 1 patient who received 2 drug chemotherapy had SD. Two of these patients underwent surgery, and other 2 underwent definitive chemoradiation. One of 3 patients who achieved PR underwent surgical resection; the other 2 remained unresectable in view of the persistent intradural extension and infratemporal fossa involvement. One patient who had SD could undergo surgery in view of clearance of infraatemporal fossa. Recent follow-up shows that 3 out of these 4 patients are alive. Conclusion: This indicates that there may be a role of induction chemotherapy in converting potentially unresectable tumors to resectable disease that could produce better outcomes in carcinoma EAC.
Publication History
Article published online:
12 July 2021
© 2015. Indian Society of Medical and Paediatric Oncology. This is an open access article published by Thieme under the terms of the Creative Commons Attribution-NonDerivative-NonCommercial-License, permitting copying and reproduction so long as the original work is given appropriate credit. Contents may not be used for commercial purposes, or adapted, remixed, transformed or built upon. (https://creativecommons.org/licenses/by-nc-nd/4.0/.)
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India
Abstract
Background:
Carcinoma of external auditory canal (EAC) is a very rare malignancy with surgical resection as the main modality of treatment. The outcomes with nonsurgical modalities are very dismal. We present a retrospective analysis of 4 patients evaluating the role of neoadjuvant chemotherapy in technically unresectable cancers.
Materials and Methods:
This is a retrospective analysis of 4 patients from our institute from 2010 to 2014 with carcinoma EAC who were deemed unfit for surgery due to extensive disease involving occipital bone with soft tissue infiltration (n = 2), temporal dura (n = 1), left temporal lobe, and extensive soft tissue involvement (n = 1). All these patients received neoadjuvant chemotherapy with docetaxel, cisplatin and 5 fluorouracil (n = 3) and paclitaxel and cisplatin (n = 1).
Results:
Response evaluation showed a partial response (PR) in 3 and stable disease (SD) in 1 patient by Response Evaluation Criteria in Solid Tumors criteria. All 3 patients who received 3 drug chemotherapy had PR while 1 patient who received 2 drug chemotherapy had SD. Two of these patients underwent surgery, and other 2 underwent definitive chemoradiation. One of 3 patients who achieved PR underwent surgical resection; the other 2 remained unresectable in view of the persistent intradural extension and infratemporal fossa involvement. One patient who had SD could undergo surgery in view of clearance of infraatemporal fossa. Recent follow-up shows that 3 out of these 4 patients are alive.
Conclusion:
This indicates that there may be a role of induction chemotherapy in converting potentially unresectable tumors to resectable disease that could produce better outcomes in carcinoma EAC.
INTRODUCTION
Carcinoma of EAC is a very rare malignancy with an incidence of 1-6 cases per million population per year.[1,2,3] Squamous carcinoma is the most frequent neoplasm which is about four times more common than basal carcinomas.[1] These tumors have an aggressive nature and usually present in advanced stages due to a lack of clear anatomical barriers.[4]
Depending on the stage of disease and treatment protocols, 5-year survival rates range from 10% for advanced disease to 83% for early disease.[5,6,7,8,9,10] Surgical resection is the main modality of treatment. Those tumors which are found to be unresectable and treated with nonsurgical modalities have a very dismal outcome.
Logically in such patients, reduction in size by the use of neoadjuvant chemotherapy might result in successful surgical resection. Based on the preliminary experience with induction chemotherapy in a selected population of technically unresectable tumors at our center,[11] we performed a retrospective analysis of 4 patients evaluating the role of neoadjuvant chemotherapy in technically unresectable cancers.
MATERIALS AND METHODS
We present a retrospective analysis of 4 patients with carcinoma EAC, who received neoadjuvant chemotherapy (NACT) at our center between 2010 and 2014. These data were retrieved from a prospectively maintained database, electronic medical records, and the case files of the patients.
These 4 patients belonged to a wide age group ranging from 28 to 60 years. Three of them were males and one of them was a female. One of them was a tobacco chewer, and one was a cigarette smoker and the rest 2 patients had no addictions. All 4 patients presented with swelling over temporal region along with serosanguineous discharge from the affected ear. Two of them had pain and decreased hearing in the affected ear each, and one had facial asymmetry. One out of these 4 patients had a past history of chronic supporative otitis media for which he was operated twice by modified radical mastoidectomy.
The magnetic resonance imaging findings of these patients revealed extensive disease. They were discussed in the multidisciplinary skull base clinic at our center and were considered technically unresectable and planned for induction chemotherapy in view of the following:
- Occipital bone and soft tissue infiltration (n = 2).
- Temporal dura, temporalis muscle and infratemporal fossa (n = 1) and
- Left temporal lobe and middle cranial fossa (n = 1).
These patients received neoadjuvant chemotherapy with 2 or 3 drugs consisting of combinations of a taxane (paclitaxel or docetaxel) and platinum (cisplatin or carboplatin) with or without 5-fluorouracil. The choice of regimens was decided on the patients’ performance status, creatinine clearance (as calculated by the Cockcroft-Gault formula), patient's preference, logistic, and financial constraints.[17] The chemotherapy protocol were as follows:
- Docetaxel, cisplatin and 5 fluorouracil (DCF) - docetaxel 75 mg/m2 (D1), cisplatin 75 mg/m2 (D1) and 5 fluorouracil 1000 mg/m2 (D1-D5) every 21 days with primary granulocyte colony-stimulating factor prophylaxis.
- Paclitaxel 175 mg/m2 and cisplatin 75 mg/m2 every 3 weekly.
After 2 cycles of chemotherapy, the patients were re-evaluated in the multidisciplinary skull base clinic. The response was assessed clinically by the surgeons for resectability and radiologically according to the Response Evaluation Criteria in Solid Tumors (RECIST 1.1) criteria. This assessment was carried out by the same group of surgeons who had initially assessed the patients. Those patients who were still unresectable underwent radical chemoradiation. All patients were followed up till date for progression and/or death.
RESULTS
Three of these 4 patients received 3 drug chemotherapy with TPF and one of them received 2 drug chemotherapy with paclitaxel and cisplatin. All the 4 patients received 2 cycles of chemotherapy after which all of them had a clinical response in form of decrease in pain, ear discharge, and swelling over temporal region. Radiologically, the response evaluation scan showed a partial response (PR) in 3 patients and stable disease (SD) in 1 patient by RECIST criteria. Figure 1 shows the magnetic resonance imaging (MRI) picture of a patient with an enhancing mass in the right external auditory canal and right temporal bone extending to the mastoid region before chemotherapy which decreased in size and extent after chemotherapy as depicted in Figure 2. Figure 3 shows the MRI picture of a mass in the right external auditory canal and middle ear cavity involving high infratemporal fossa (ITF) making it inoperable. As depicted in Figure 4, post chemotherapy there was no disease appreciable in the high ITF. All 3 patients who received 3 drug chemotherapy had a PR while 1 patient who received 2 drug chemotherapy had an SD. Two of these patients underwent surgery, and other 2 patients underwent definitive chemoradiation. One of the 3 patients, who achieved PR underwent surgical resection, however the other 2 patients still remained unresectable in view of persistent intradural extension and infratemporal fossa involvement though there was regression o the external auditory component of the disease. The 1 patient who had SD after 2 drug chemotherapy could undergo surgical resection as there was the clearance of disease in the infratemporal fossa.
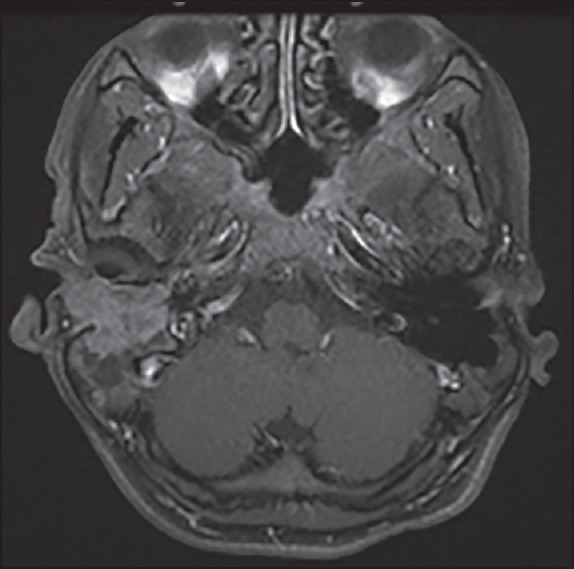
| Fig. 1 Magnetic resonance image before chemotherapy an enhancing mass in the right external auditory canal and right temporal bone involving the mastoid
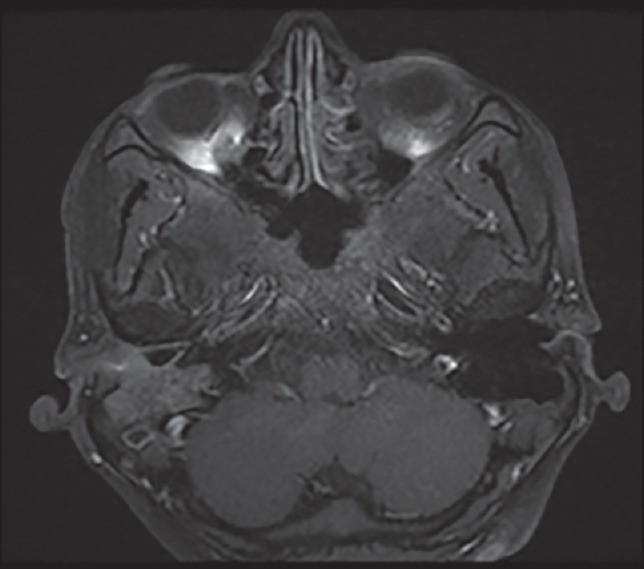
| Fig. 2 Magnetic resonance image after chemotherapy decrease in size and extent of the mass in right external auditory canal and right temporal bone involving the mastoid
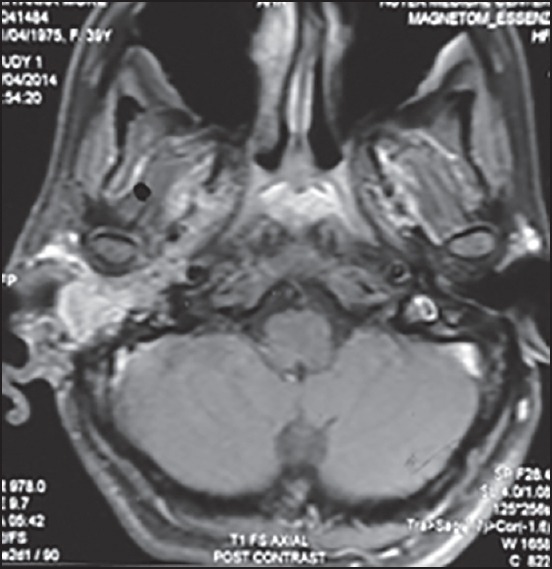
| Fig. 3 Magnetic resonance image showing a mass in right external auditory canal and middle ear cavity involving high infratemporal fossa
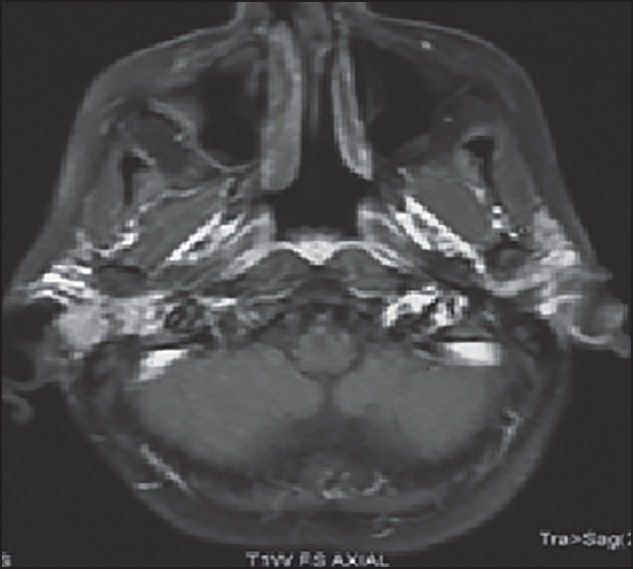
| Fig. 4 Magnetic resonance image showing decrease in size of mass in right external auditory canal and middle ear cavity with no disease appreciable in high infratemporal fossa
Recent follow-up shows that 3 out of these 4 patients are alive. Two of these patients have clinically and radiologically controlled disease. Both these patients had a PR after 3 drug chemotherapy, however, remained unresectable and underwent concurrent chemoradiation in view of the persistent intradural extension and infratemporal fossa involvement, respectively. The other 2 patients had a disease recurrence. One of these who had an SD after 2 drug chemotherapy underwent surgical resection but a right frontal region recurrence at 3 months follow-up and is presently undergoing palliative radiotherapy for the same. The other patient who had a PR after 3 drug chemotherapy underwent surgical resection followed by adjuvant chemoradiation but developed a left posterior auricular swelling at 6 months follow-up and died due to the progression of disease.
DISCUSSION
To the best of our knowledge, this is the first data documenting response to chemotherapy in carcinoma of EAC and the use of neoadjuvant chemotherapy in technically unresectable carcinoma EAC.
Carcinoma of external auditory canal (EAC) is a very rare malignancy with a global incidence of 1-6 cases per million per year.[1,2,3] These tumors have an aggressive nature with a tendency to spread early along preformed vascular and neural pathways, invading adjacent structures.[4] Due to this reason and also because the initial symptoms are similar to those of benign diseases, these tumors are usually diagnosed in advanced stages.[4]
Depending on the stage of disease and treatment protocols, 5-year survival rates range from 10% for advanced disease to 83% for early disease.[5,6,7,8,9,10]
The preferred treatment for cancer of the EAC is radical surgery. Nonsurgical modalities like definitive radiation have a 3-year disease-free survival as low as 15% and the mean survival in the palliatively irradiated group was 3.6 months.[12] It may be useful to make unresectable patients resectable thereby improving outcome in such patients.
Induction chemotherapy has been used in head and neck cancer with promising results.[13,15] Squamous cell carcinoma of head and neck cancer responds very well to chemotherapy, but the differential response has also been noted for different sites of disease. The retrospective analysis of the prospectively collected data at our hospital from 2008 to 2012 suggested that out of 862 patients referred for neoadjuvant chemotherapy, the sites were oral cavity in 83.6%, maxilla in 4.8%, larynx in 3.8%, laryngopharynx in 0.9%, and hypopharynx in 8.2%.[16] However, response to chemotherapy has not been documented for carcinoma of EAC.
We have used neoadjuvant chemotherapy previously in the technically unresectable head and neck cancers at our hospital which led to successful resection and improved overall survival (OS). A retrospective analysis of 721 patients with stage IV technically unresectable oral cavity cancer who received neoadjuvant chemotherapy showed that 310 patients (43%) had sufficient reduction in tumor size to undergo surgical resection. The locoregional control rate at 24 months (32% vs. 15%) and the median estimated OS (19.6 months vs. 8.16 months) significantly improved in patients undergoing surgery as compared to those treated with nonsurgical treatment.[11] Similarly, in another study of 41 patients with locally advanced technically unresectable maxillary carcinoma who were treated with induction chemotherapy, the response evaluation depicted SD in 43.9% and PR in 39%. After induction, surgery could be performed in 29.3% of patients. Overall, the median progression-free survival was 10 months and OS at 36 months was 35%.[17]
Based on these results, we used neoadjuvant chemotherapy in patients with carcinoma of EAC to patients who were considered unresectable in view of disease involving occipital bone, temporal dura, temporalis muscle, infratemporal fossa, left temporal lobe, and extensive soft tissue infiltration.
Two of these 4 patients became resectable. All these patients would have otherwise undergone definitive chemoradiation or palliative radiotherapy or chemotherapy. Licitra et al. also showed that the use of chemotherapy in resectable oral cavity cancers reduced the amount of demolitive surgery (31% vs. 52% in the control group) without detecting any increased number of positive surgical margins, and lessened postoperative radiotherapy (33% vs. 46%) possibly due to the presence of new safe revised margin with downstaging of the tumor.[13] Hence, the use of induction chemotherapy definitely seems to make a proportion of these patients with borderline unresectable tumors resectable which may improve the overall outcome and lead to an increase in the OS.
All 3 patients who received 3 drug chemotherapy achieved a PR while the 1 patient who received 2 drug chemotherapy had an SD. Two of these 4 patients (1 after 3 drug chemotherapy and 1 after 2 drug chemotherapy) underwent successful resection after induction chemotherapy. Those who were still unresectable after induction chemotherapy received the concurrent chemoradiation.
In light of these studies and the results of the 4 patients in our case series, our approach of using induction chemotherapy could convert potentially unresectable tumors to a resectable disease that could produce better outcomes.
CONCLUSION
The results of these 4 patients suggest that carcinoma EAC responds to chemotherapy. The use of induction chemotherapy in carcinoma EAC may have a role in making a proportion of borderline unresectable patients operable.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES

| Fig. 1 Magnetic resonance image before chemotherapy an enhancing mass in the right external auditory canal and right temporal bone involving the mastoid

| Fig. 2 Magnetic resonance image after chemotherapy decrease in size and extent of the mass in right external auditory canal and right temporal bone involving the mastoid

| Fig. 3 Magnetic resonance image showing a mass in right external auditory canal and middle ear cavity involving high infratemporal fossa

| Fig. 4 Magnetic resonance image showing decrease in size of mass in right external auditory canal and middle ear cavity with no disease appreciable in high infratemporal fossa


 PDF
PDF  Views
Views  Share
Share

