Neutrophil-to-lymphocyte Ratio: A Surrogate Marker for Prognosis of Oral Squamous Cell Carcinoma
CC BY-NC-ND 4.0 ? Indian J Med Paediatr Oncol 2018; 39(01): 8-12
DOI: DOI: 10.4103/ijmpo.ijmpo_2_16
Abstract
Context:?Recent studies show that enzymatic contents of the neutrophil granules have a remarkable ability to modulate the tumor microenvironment by causing apoptosis of T-lymphocytes which leaves the host's cell-mediated immunity at stake. The preoperative neutrophil to lymphocyte ratio (NLR) is considered to be an indicator of the immune status of the patients with oral squamous cell carcinoma (OSCC), which will thereby help in predicting the course of the disease.?Aims:?The aim is to assess the NLR and histopathological prognostic factors pertinent to infiltration of the surrounding structures and correlate them with the clinical prognostic outcomes of OSCC. Settings and Design: This retrospective study involved the retrieval of formalin-fixed, paraffin-embedded, hematoxylin, and eosin-stained sections of 55 cases of OSCC from the departmental archives from 2006 to 2014.?Subjects and Methods:?Grading of each case was done by Bryne's grading system. The preoperative complete blood counts, relevant case history, and clinical data of the patients involved in the study were collected from the institutional medical records. The NLR was calculated by dividing the serum neutrophil count by the serum lymphocyte count.?Statistical Analysis Used:?The median NLR was compared between the controls and OSCC cases using Wilcoxon-signed rank test, and the Kaplan-Meier survival analysis was carried out to predict the survival and recurrence status of OSCC.?Results:?Higher NLR was seen in lymph node and margin-involved cases and also in patients who had tumor recurrence. Kaplan-Meier survival analysis showed that the mean survival dropped from 26 to 4.5 months when NLR ?5 (P?= 0.052).?Conclusion:?Neutrophil-to-lymphocyte ratio can be used as a prognosticator of survival, recurrence, lymph node status, and margin status in OSCC.?
Keywords
Cannibalism - interleukin-8 - neutrophil - neutrophil to lymphocyte ratio - oral squamous cell carcinoma - prognosis
Publication History
23 June 2021 (online)
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India
Abstract
Context:?Recent studies show that enzymatic contents of the neutrophil granules have a remarkable ability to modulate the tumor microenvironment by causing apoptosis of T-lymphocytes which leaves the host's cell-mediated immunity at stake. The preoperative neutrophil to lymphocyte ratio (NLR) is considered to be an indicator of the immune status of the patients with oral squamous cell carcinoma (OSCC), which will thereby help in predicting the course of the disease.?Aims:?The aim is to assess the NLR and histopathological prognostic factors pertinent to infiltration of the surrounding structures and correlate them with the clinical prognostic outcomes of OSCC. Settings and Design: This retrospective study involved the retrieval of formalin-fixed, paraffin-embedded, hematoxylin, and eosin-stained sections of 55 cases of OSCC from the departmental archives from 2006 to 2014.?Subjects and Methods:?Grading of each case was done by Bryne's grading system. The preoperative complete blood counts, relevant case history, and clinical data of the patients involved in the study were collected from the institutional medical records. The NLR was calculated by dividing the serum neutrophil count by the serum lymphocyte count.?Statistical Analysis Used:?The median NLR was compared between the controls and OSCC cases using Wilcoxon-signed rank test, and the Kaplan-Meier survival analysis was carried out to predict the survival and recurrence status of OSCC.?Results:?Higher NLR was seen in lymph node and margin-involved cases and also in patients who had tumor recurrence. Kaplan-Meier survival analysis showed that the mean survival dropped from 26 to 4.5 months when NLR ?5 (P?= 0.052).?Conclusion:?Neutrophil-to-lymphocyte ratio can be used as a prognosticator of survival, recurrence, lymph node status, and margin status in OSCC.
Keywords
Cannibalism - interleukin-8 - neutrophil - neutrophil to lymphocyte ratio - oral squamous cell carcinoma - prognosis
Introduction
Squamous cell carcinoma is the most common neoplasm of the oral cavity accounting for at least 90% of all oral malignancies. The outcome of the disease is influenced by multiple factors modulating tumor as well as host response.[1]
Tumor-associated inflammatory response has been long recognized as a vital constituent of the spectrum of factors that curtail neoplastic cell progression and directly influence the grade and prognosis of the disease. The cells commonly observed to be a part of the histology of various malignant tumors include macrophages, neutrophils, mast cells, natural killer (NK) cells, and lymphocytes. Neutrophils, which represent 50%?70% of the total circulating leukocytes, have been found to comprise a significant portion of the leukocytic infiltrate in a wide variety of human cancers.[2]
An elevation in blood neutrophil-to-lymphocyte ratio (NLR) is considered to be a marker, which predisposes the tumor to proliferation and metastasis through inhibition of apoptosis, promotion of angiogenesis, and damage of DNA. Recent evidence has shown that a high preoperative blood NLR is associated with poor outcome in various malignancies, including esophageal cancer, hepatocellular, and breast cancers. However, no studies have been reported regarding the validity of the role of NLR as a marker for predicting the prognosis of oral squamous carcinoma.[2]
Therefore, this study aimed to estimate the NLR in patients with oral squamous cell carcinoma (OSCC) and in normal individuals and to find its association with lymph node status, margin status, recurrence, and survival in patients with OSCC.
Subjects and Methods
After obtaining the necessary approval from the institutional ethics committee, 55 formalin-fixed, paraffin-embedded, and hematoxylin and eosin-stained sections of cases of OSCC were retrieved from the departmental archives. Grading of each case was done by Bryne's grading system of invasive tumour front.[3] The preoperative complete blood counts, relevant case histories, and clinical data of the patients involved in the study were collected from the institutional medical records. The NLR was calculated by dividing the serum neutrophil count by the serum lymphocyte count.
Results
The results of the present study showed that the NLR of histopathologically-proven cases of OSCC showed a significantly higher median of 2.7 (P?< 0 href="https://www.thieme-connect.com/products/ejournals/html/10.4103/ijmpo.ijmpo_2_16#JR_4" xss=removed>4] using one sample Wilcoxon-signed rank test. Compared to the well-differentiated OSCC (n?- 24, median of 2.44) NLR was found to be increased in poorer grades of tumor with moderately-differentiated cases (n?- 18) having a median of 3.2 and poorly differentiated cases (n?- 11) having a median of 2.9 (Kruskal?Wallis test, P = 0.399). Similarly, cases with recurrence showed a higher NLR of 2.7 as compared to non-recurrent cases whose median was 2.4 (P?= 0.431). NLR was slightly higher though not statistically significant in cases where the surgical margins were involved by tumor (median of 2.9 vs. 2.7). Patients with lymph nodes involved by tumor had a higher NLR (median of 2.7) than those who had tumor-free lymph nodes (median = 2.4) (P?= 0.908) [Figure 1]. The Kaplan-Meier survival analysis showed that when the NLR was> 5, the survival dropped to a mean of 4.5 months as compared to 26.83 months for the NLR values <5 class="i" xss=removed>P?= 0.052) [Figure 2].
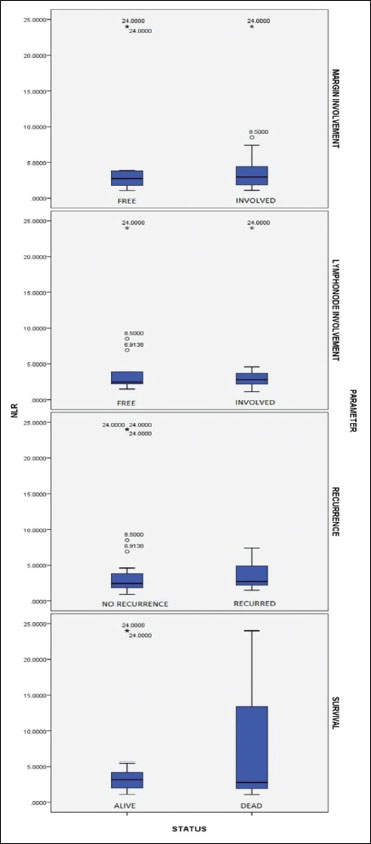
|?Figure. 1Box-and-whisker plot showing margin status, lymph node status, recurrence, and survival in oral squamous cell carcinoma
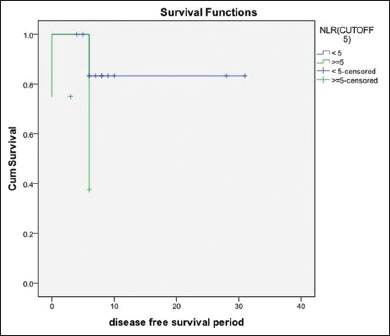
|?Figure. 2Kaplan-Meier survival curve showing significantly lower survival when neutrophil to lymphocyte ratio is >5
Discussion
Inflammation is an integral feature in tumor biology as tumor-associated inflammation is considered to be an important factor that regulates tumor progression. Neutrophils are the most common type of acute inflammatory cells present in the peripheral blood. They are constantly on a ?ready to respond? mode to chemotactic stimuli, thereby helping the host to ward off the infection. These cells account for about 60% of the total leukocyte population but are short-lived. Their life could be modulated in certain specific micro environments like the tumor microenvironment, in which they are educated in favor of tumor progression. During this process, they release certain enzymes that modify the extracellular matrix (ECM) providing a path for their easy migration to the site of microbial insult/injury and kill them, which is a part of host defense. The B and T lymphocytes and NK cells are in addition the more specific and efficient major drivers of chronic inflammation and are a part of cell-mediated immunity.
The ratio between the serum neutrophil count and the serum lymphocyte count is termed as NLR. It is considered to be an independent prognostic indicator in hepatocellular carcinoma, colorectal carcinoma and various ovarian tumors.[5],[6] NLR may also be regarded as an indicator of the proportion of cell-mediated or innate immunity. A higher NLR is indicative of a lagging cell-mediated immunity and vice versa.
Neutrophils and macrophages are credited with the innate ability to release interleukin (IL)-8. Under normal physiological conditions, the function of IL-8 is to attract neutrophils to the site of injury along with the help of other inflammatory mediators like tumor necrosis factor-? and IL-1?, and prevent the wound from further microbial insult and promote healing. Once the infection is cleared, or the wound is healed, IL-8 secretion stops, attenuating the influx of neutrophils and the consequential inflammatory responses.[7]
Tumor cells are also known to ectopically secrete IL-8 in a much higher concentration attracting increasing numbers of neutrophils and modulating them to their favor. A direct correlation between the tumor cell lines secreting increased levels of IL-8 and their metastatic potential has been shown for many tumor cell types.[8],[9] An in vivo study by Trellakis?et al. has shown that the level of IL-8 secreted by the metastatic cell lines were higher than in their poorly metastatic counterparts.[10] IL-8 is also considered to be an autocrine growth and motility factor of many tumor cell lines, thus the tumor cells which respond to this ectopically secreted IL-8 are said to have an advantage of growth and progression.[7]
Neutrophil tumor cell cannibalism
The reasons for ectopic and excessive production of IL-8 by the tumor cells may be explained by the concept of neutrophil tumor cell cannibalism. As the tumor progresses toward a poorer histological grade, tumor cells lose cohesion within the tumor and show less interaction with the surrounding connective tissue stroma. It is quite conceivable that the dedifferentiated tumor cells could indulge in cannibalism resulting in neutrophil phagocytosis for its survival. When this happens, a horizontal DNA transfer occurs from the neutrophils to the tumor cells leading to the acquisition of the properties of neutrophils by the neoplastic cell.[11] This leads to the ectopic production of cytokines and expression of receptors of neutrophils by the tumor cells. Tumor cells may thus stimulate the production of neutrophils as well as induce their chemotaxis to the tumor site. This is supported by?in vitro?studies on the role of polymorphonuclear neutrophils (PMN's) in tumor progression where human head and neck squamous cell carcinoma tumor cell lines modulate granulocyte immunobiology by directly recruiting the PMN's, prolonging their survival and promoting their inflammatory activity.[12]
Lymphocytopenia
The cytolytic activity of lymphocytes, activated T cells and NK cells, is said to be suppressed by neutrophils, and the degree of suppression is closely associated with number of neutrophils.[13] Tumor-derived factors are found to induce death of immune cells at the site of the tumor and also in the peripheral blood. Various studies have reported a higher frequency of death of immune cells in the blood of cancer patients compared to the controls that could be explained by the significantly higher expression of Fas in patients in advanced stages of the disease and with increased tumor load. Thus, spontaneous apoptosis of CD8 T cells leads to rapid lymphocyte turnover and a decrease in an absolute number of T cell subsets which is seen in most of the advanced and poorly-differentiated cases.[14] The relative decrease in lymphocytes in comparison to the abnormally high neutrophils could also be considered as a reason for higher NLR.
Neutrophil to lymphocyte ratio and tumor differentiation
NLR is increased in initial tumors as a host response to the recognition of the ?foreign nature? of tumor in the connective tissue zone. In advanced stages there is also suppression of lymphocytes further leading to relative increase in NLR. This is well supported in our study as a progressive increase in the NLR is evident in cases showing poorer differentiation. While it can detect a trend in dedifferentiation, it may not be effective in delineating the moderately and poorly differentiated grades of OSCC. Poorly-differentiated cases have a greater genomic instability which could be attributed to the increase in the infiltration of neutrophils. As the tumor matures, it integrates the function of neutrophils with it to further increase the proportion of neutrophils. Haqqani?et al. have proved that the reactive nitrogen oxygen species that are produced by the activated neutrophils in the tumor milieu are to an extent responsible for the accumulation of mutations that are responsible for tumor progression.[15]
Neutrophil to lymphocyte ratio and margins, lymph nodes, and recurrence
Our results showed a higher NLR in cases with involvement of surgical margins and lymph nodes and in those who had recurrence. This could be attributed to the fact that neutrophils perform two important functions, (i) angiogenesis, ii) production of reactive oxygen species (ROS).
Neutrophil elastase (NE), a type of serine protease is synthesized in large quantities by the neutrophils during the myelo-monocytic stages of bone marrow development. Later in life, they synthesize metalloproteinases such as neutrophil collagenase (matrix metalloproteinase [MMP]-8) and gelatinase B (MMP-9), which is stored within the cytoplasmic granules. Neutrophils are said to have an adapted strategy for rapid dispersal of these enzymes to the phagolysosome while sequestering them from vital parts of the cell. The killing of the microorganisms is assisted by the rapid transport of ROS, defensins and serine proteases. Small amounts of proteinases are also released into the ECM in quantum micro-bursts to transiently overwhelm their inhibitors to accomplish focused proteolysis.[13] Thus, the ECM components are hydrolyzed by the neutrophilic contents [12] and this remodeled ECM offers decreased resistance for the tumor cells to traverse thereby leading to greater chances of margin involvement or lymph node metastases.
Angiogenesis
Neutrophils are considered to be a major source of vascular endothelial growth factor (VEGF) which promotes angiogenesis that in turn promotes metastasis. Various studies on Hepatocellular carcinoma have shown a positive correlation between higher levels of VEGF and tumor recurrence.[6],[13] Once the neutrophils reach the tumor, they release the enzyme NE within the tumor milieu. NE activates latent proteases which can then cleave and inactivate plasminogen activator inhibitor 1, which is the natural inhibitor of plasmin. During the process of remodeling, embedded growth factors like basic fibroblast growth factor which is also a potent angiogenic factor and a growth factor for endothelial cells is released.[7] As the neutrophils traverse through the newly remodeled ECM, it makes way for the endothelial cells to move to the site of tumor, thus promoting angiogenesis and sustenance of tumor toward dedifferentiation.
Role of reactive oxygen species ? decreased cell matrix interaction
The neutrophilic enzymes used in the production of ROS are nicotinamide adenine dinucleotide phosphate oxidase and myeloperoxidase. The NADPH oxidase reduces O2 to superoxide anion, which is converted to hydrogen peroxide (H2O2). The enzyme myeloperoxidase which is present at high concentrations in neutrophils converts H2O2 to hypochlorous acid (HOCl) in the presence of chloride ions. HOCl is a potent modifier of several proteins of the ECM. Thus, these further modifications bring about a decrease in the cell-matrix interaction.[7]
Activation of latent proteases by NE also results in decreased cell ? cell interactions. Thus, the tumor cells can dissociate easily from the tumor mass and move to different sites leading to loco-regional recurrence.[7]
In summary, tumor de-differentiation is a vicious cycle. The dedifferentiation induces innate production of IL-8 because of integration of neutrophil DNA with that of tumor cells. Furthermore, dedifferentiated tumor cells induce lymphocyte apoptosis mediated by Fas ligands, collectively increasing the NLR. Increased neutrophils produce ROS which induces genomic instability resulting in further de-differentiation. Neutrophil products like ROS, NE, MMP 8 and VEGF hasten the ECM degradation and promote angiogenesis, thus accounting for the positive correlation of NLR with metastasis and poor survival [Figure 3].
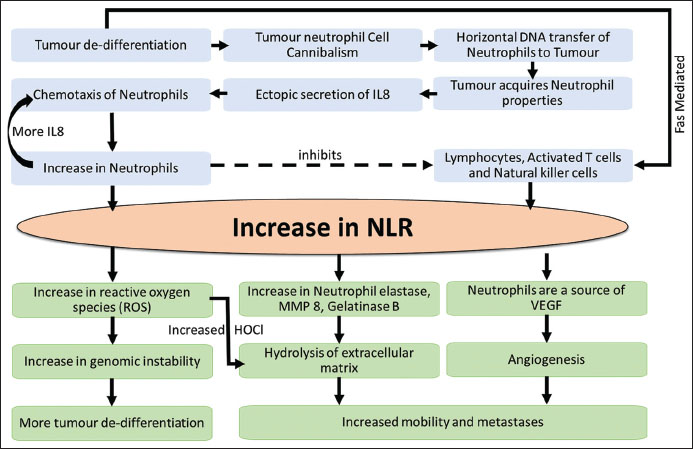
|?Figure. 3Pathogenesis of neutrophil to lymphocyte ratio in oral squamous cell carcinoma
We found a significantly high preoperative NLR in OSCC patients who had a poor survival rate. Higher NLR was also found in cases that showed the involvement of surgical margins and lymph nodes. Patients with higher NLR showed a higher tumor recurrence rate than those with a lower NLR. Thus, NLR can be used as a feasible, cost-effective, and potential biomarker to preoperatively identify patients with poor prognosis and adverse tumor biology.
Conflict of Interest
There are no conflicts of interest.
References
- assano J, Regateiro FS, Janu?rio G, Ferreira A.?Oral squamous cell carcinoma: Review of prognostic and predictive factors. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2006; 102: 67-76
- ang J, Jia Y, Wang N, Zhang X, Tan B, Zhang G.?et al.?The clinical significance of tumor-infiltrating neutrophils and neutrophil-to-CD8+ lymphocyte ratio in patients with resectable esophageal squamous cell carcinoma. J Transl Med 2014; 12: 7
- ryne M, Stromme H, Lilleng R, Stene T, Bang G, Dabelsteen E, Bryne M.?New malignancy grading is a better prognostic indicator than Broder's grading in oral squamous cell carcinoma. J Oral Pathol Med 1989; 18: 432-7
- ewis SM, Bain BJ, Bates I.?Basic haematological techniques. In: Practical Haematology. New Delhi: Reed Elsevier India Private Limited 2007; 10: 25-59
- haraiha RZ, Halazun KJ, Mirza F, Port JL, Lee PC, Neugut AI.?et al.?Elevated preoperative neutrophil:lymphocyte ratio as a predictor of postoperative disease recurrence in esophageal cancer. Ann Surg Oncol 2011; 18: 3362-9
- iao WK, Chen D, Li SQ, Fu SJ, Peng BG, Liang LJ.?et al.?Prognostic significance of neutrophil-lymphocyte ratio in hepatocellular carcinoma: A meta-analysis. BMC Cancer 2014; 14: 117
- e LarcoJE, Wuertz BR, Furcht LT. The potential role of neutrophils in promoting the metastatic phenotype of tumors releasing interleukin-8?Clin Cancer Res?2004; 10:?4895-900
- ingh RK, Gutman M, Radinsky R, Bucana CD, Fidler IJ.?Expression of interleukin 8 correlates with the metastatic potential of human melanoma cells in nude mice. Cancer Res 1994; 54: 3242-7
- e LarcoJE, Wuertz BR, Rosner KA, Erickson SA, Gamache DE, Manivel JC.?et al?A potential role for interleukin-8 in the metastatic phenotype of breast carcinoma cells. Am J Pathol 2001; 158: 639-46
- Trellakis S, Bruderek K, Dumitru CA, Gholaman H, Gu X, Bankfalvi A.?et al.?Polymorphonuclear granulocytes in human head and neck cancer: Enhanced inflammatory activity, modulation by cancer cells and expansion in advanced disease. Int J Cancer 2011; 129: 2183-93
- Sarode SC, Sarode GS.?Neutrophil-tumor cell cannibalism in oral squamous cell carcinoma. J Oral Pathol Med 2014; 43: 454-8
- Houghton AM.?The paradox of tumor-associated neutrophils: Fueling tumor growth with cytotoxic substances. Cell Cycle 2010; 9: 1732-7
- Fang HY, Huang XY, Chien HT, Chang JT, Liao CT, Huang JJ.?et al.?Refining the role of preoperative C-reactive protein by neutrophil/lymphocyte ratio in oral cavity squamous cell carcinoma. Laryngoscope 2013; 123: 2690-9
- Sinha S, Whiteside TL.?Immune responses to cancer: Are they potential biomarkers of prognosis?. Front Oncol 2013; 3: 107
- Haqqani AS, Sandhu JK, Birnboim HC.?Expression of interleukin-8 promotes neutrophil infiltration and genetic instability in mutatect tumors. Neoplasia 2000; 2: 561-8
Address for correspondence
Publication History
23 June 2021 (online)
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India

|?Figure. 1Box-and-whisker plot showing margin status, lymph node status, recurrence, and survival in oral squamous cell carcinoma

|?Figure. 2Kaplan-Meier survival curve showing significantly lower survival when neutrophil to lymphocyte ratio is >5

|?Figure. 3Pathogenesis of neutrophil to lymphocyte ratio in oral squamous cell carcinoma
References
- Massano J, Regateiro FS, Janu?rio G, Ferreira A.?Oral squamous cell carcinoma: Review of prognostic and predictive factors. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2006; 102: 67-76
- Wang J, Jia Y, Wang N, Zhang X, Tan B, Zhang G.?et al.?The clinical significance of tumor-infiltrating neutrophils and neutrophil-to-CD8+ lymphocyte ratio in patients with resectable esophageal squamous cell carcinoma. J Transl Med 2014; 12: 7
- Bryne M, Stromme H, Lilleng R, Stene T, Bang G, Dabelsteen E, Bryne M.?New malignancy grading is a better prognostic indicator than Broder's grading in oral squamous cell carcinoma. J Oral Pathol Med 1989; 18: 432-7
- Lewis SM, Bain BJ, Bates I.?Basic haematological techniques. In: Practical Haematology. New Delhi: Reed Elsevier India Private Limited 2007; 10: 25-59
- Sharaiha RZ, Halazun KJ, Mirza F, Port JL, Lee PC, Neugut AI.?et al.?Elevated preoperative neutrophil:lymphocyte ratio as a predictor of postoperative disease recurrence in esophageal cancer. Ann Surg Oncol 2011; 18: 3362-9
- Xiao WK, Chen D, Li SQ, Fu SJ, Peng BG, Liang LJ.?et al.?Prognostic significance of neutrophil-lymphocyte ratio in hepatocellular carcinoma: A meta-analysis. BMC Cancer 2014; 14: 117
- De LarcoJE, Wuertz BR, Furcht LT. The potential role of neutrophils in promoting the metastatic phenotype of tumors releasing interleukin-8?Clin Cancer Res?2004; 10:?4895-900
- Singh RK, Gutman M, Radinsky R, Bucana CD, Fidler IJ.?Expression of interleukin 8 correlates with the metastatic potential of human melanoma cells in nude mice. Cancer Res 1994; 54: 3242-7
- De LarcoJE, Wuertz BR, Rosner KA, Erickson SA, Gamache DE, Manivel JC.?et al?A potential role for interleukin-8 in the metastatic phenotype of breast carcinoma cells. Am J Pathol 2001; 158: 639-46
- ;Trellakis S, Bruderek K, Dumitru CA, Gholaman H, Gu X, Bankfalvi A.?et al.?Polymorphonuclear granulocytes in human head and neck cancer: Enhanced inflammatory activity, modulation by cancer cells and expansion in advanced disease. Int J Cancer 2011; 129: 2183-93
- ;Sarode SC, Sarode GS.?Neutrophil-tumor cell cannibalism in oral squamous cell carcinoma. J Oral Pathol Med 2014; 43: 454-8
- ;Houghton AM.?The paradox of tumor-associated neutrophils: Fueling tumor growth with cytotoxic substances. Cell Cycle 2010; 9: 1732-7
- ;Fang HY, Huang XY, Chien HT, Chang JT, Liao CT, Huang JJ.?et al.?Refining the role of preoperative C-reactive protein by neutrophil/lymphocyte ratio in oral cavity squamous cell carcinoma. Laryngoscope 2013; 123: 2690-9
- ;Sinha S, Whiteside TL.?Immune responses to cancer: Are they potential biomarkers of prognosis?. Front Oncol 2013; 3: 107
- ;Haqqani AS, Sandhu JK, Birnboim HC.?Expression of interleukin-8 promotes neutrophil infiltration and genetic instability in mutatect tumors. Neoplasia 2000; 2: 561-8


 PDF
PDF  Views
Views  Share
Share

