Obesity-related Cancers: The Coming Epidemic
CC BY-NC-ND 4.0 · Indian J Med Paediatr Oncol 2020; 41(03): 328-334
DOI: DOI: 10.4103/ijmpo.ijmpo_117_20
Abstract
The world is in the grip of an obesity pandemic, with tripling of obesity rates since 1975; it is predicted that one-third of people on Earth will be obese by 2025. The health consequences of obesity are primarily thought to be related to cardiometabolic disorders such as diabetes and cardiovascular diseases. It is less well appreciated that obesity has been related to at least 13 different cancers and in future, (with increasing control over tobacco misuse and infections), obesity will be the main cause of cancers. While this is an area of active research, there are large gaps in the definition of what is an obesity related cancer (JRC) and more importantly, what are the underlying mechanisms. To an extent, this is due to the controversy on what constitutes “unhealthy obesity” which is further related to the causes of obesity. This narrative review examines the causes and measurement of obesity, the types of obesity-related cancers and possible mechanisms. The information has wide implications ranging from prevention, screening, prognosis and therapeutic strategies. Obesity related cancers should be an area of high-priority research. Oncologists can contribute by spreading awareness and instituting management measures for individual patients in their care.
Keywords
Carbohydrate-insulin model - gut microbiome - hyperinsulinemia - obesity - obesity-related cancers - sarcopenic obesityPublication History
Received: 31 March 2020
Accepted: 26 May 2020
Article published online:
28 June 2021
© 2020. Indian Society of Medical and Paediatric Oncology. This is an open access article published by Thieme under the terms of the Creative Commons Attribution-NonDerivative-NonCommercial-License, permitting copying and reproduction so long as the original work is given appropriate credit. Contents may not be used for commercial purposes, or adapted, remixed, transformed or built upon. (https://creativecommons.org/licenses/by-nc-nd/4.0/.)
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India
Abstract
The world is in the grip of an obesity pandemic, with tripling of obesity rates since 1975; it is predicted that one-third of people on Earth will be obese by 2025. The health consequences of obesity are primarily thought to be related to cardiometabolic disorders such as diabetes and cardiovascular diseases. It is less well appreciated that obesity has been related to at least 13 different cancers and in future, (with increasing control over tobacco misuse and infections), obesity will be the main cause of cancers. While this is an area of active research, there are large gaps in the definition of what is an obesity related cancer (JRC) and more importantly, what are the underlying mechanisms. To an extent, this is due to the controversy on what constitutes “unhealthy obesity” which is further related to the causes of obesity. This narrative review examines the causes and measurement of obesity, the types of obesity-related cancers and possible mechanisms. The information has wide implications ranging from prevention, screening, prognosis and therapeutic strategies. Obesity related cancers should be an area of high-priority research. Oncologists can contribute by spreading awareness and instituting management measures for individual patients in their care.
Keywords
Carbohydrate-insulin model - gut microbiome - hyperinsulinemia - obesity - obesity-related cancers - sarcopenic obesityIntroduction
The world of obesity is viewed through the lens of cardiometabolic disorders. It is less well appreciated that obesity confers significant risk of specific type of cancers and that obesity will be the leading cause of cancer in the coming years.
The Obesity Pandemic
The world is facing an obesity pandemic. The World Health Organization estimates that obesity rates across the globe have tripled since 1975, and in 2016, more than 1.9 billion adults were overweight and of these, over 650 million were obese. The Gulf region has been particularly affected by this epidemic, with an estimated 30%–40%-of the population being overweight or obese. Based on 2016 data, the CIA World Factbook identifies that Kuwait is the “fattest” country in the Gulf with almost 40%-of population being obese; Oman at 27%-has the lowest percentage of obese adults in the GCC (ranked 29th globally).
The cardiometabolic risks associated with obesity, such as Type 2 diabetes mellitus (T2DM), hypertension, fatty liver (nonalcoholic fatty liver disease [NAFLD]), hypertension, and coronary artery disease, are well known. It is less well appreciated that obesity increases the risk of several types of cancer.
Obesity-Related Cancers
It is estimated that 9%-of the cancer burden in North America, Europe, and Middle East in 2013 was obesity related.[1] Mendelian randomization studies have placed the risk of obesity-related cancers (JRCs) even higher.[2] This prevalence is likely to have grown since, especially after control of competing causes of cancer such as smoking and infection. In 2015, tobacco smoking contributed to the largest proportion of cancer cases in the UK, closely followed by overweight/obesity, accounting for 15.1%-and 6.3%, respectively.[3] Obesity-related cancers accounted for nearly 43.5%-of total direct cancer care expenditures, estimated at $35.9 billion in 2015 in USA alone.[4] The trend in increasing obesity is more marked in Saudi Arabia than in India. This has resulted in a disproportionately higher level of JRCs in Saudi Arabia (4%–9%) as compared to a more modest 0.2%–1.2%-in India.[1]
Increasing childhood obesity is a matter of grave concern as it has shifted the burden of cancer to younger age groups.[5] In addition, being overweight before the age of 40 increases the risks of various JRCs by 15%. The study from Bergen (Norway) showed increased risk of cancers of the endometrium (by 70%), renal cancer in males (by 58%), and colon cancer in male (by 29%).[6]
Defining Obesity
The cause (s) of obesity and the current epidemic is a matter of controversy. The classical energy imbalance (“calorie in, calorie out”) model attributes obesity to eating more and moving less (“gluttony and sloth”). This has been challenged by the “carbohydrate-insulin” model which suggests that the components of the Western diet such as highly refined carbohydrates (sugar and fructose) and processed food (including some seed oils and artificial sweeteners) spike insulin levels, which leads to fat storage and continued hunger [Figure 1].[7],[8] Some researchers blame the governmental advice in the seventies to cut down on fat and eat more carbohydrates for this epidemic. The field is further clouded by difficulties in defining and quantifying “unhealthy obesity” as it appears that not all obese adults have metabolic complications.
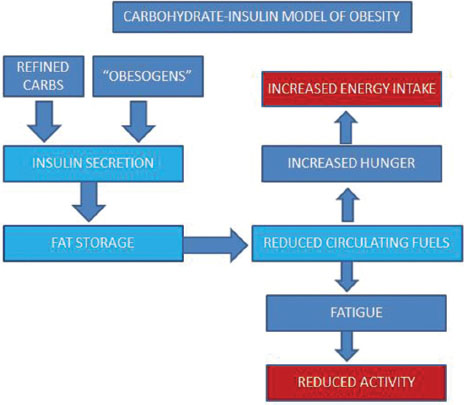
| Figure 1:The carbohydrate-insulin model of obesity
The standard method of quantifying obesity is by the body mass index (BMI) (also known as the Quetelet
index), which is weight (in kilograms) divided by the height (in meters) squared. Healthy BMI has been defined as a
value between 18 and 25; overweight is more than 25, and obese more than 30. Although a convenient method of
measurement, this index suffers from serious deficiencies. This index cannot, for instance, distinguish between fat
and lean weight. A muscular man will be classified as overweight or worse (for instance, Dwyane Johnson
It has been shown that postmenopausal women with a normal BMI but with higher body fat levels (as measured by the gold standard of dual-energy X-ray absorptiometry or DXA; other methods include hydrostatic weighing; bioelectrical impedance analysis; air displacement plethysmography; and bioimpedance spectroscopy; these can be combined together to generate multicompartment models) are at elevated risk for breast cancer.[11] These are hold the middle ground – “the metabolic obesity in normal weight”. On the other end of the spectrum, there are people who are thin but diabetic (“TOFI”, thin outside, fat inside) [Figure 2].[12] This variation is partly explained by the idea that fat storage in subcutaneous tissue is essentially safe (to a limit), but when it spills over and stored ectopically (in muscle, and especially in the liver), leads to insulin resistance, hyperinsulinemia (HI), hyperglycemia, and diseases associated with the metabolic syndrome.[13] WHR is one way to measure the ectopic fat stored in VAT and correlates better with metabolic syndrome than BMI;[14] other methods such as relative fat mass (RFM) have been proposed to overcome limitations of BMI.[15] RFM correlates closely with the gold standard DXA scan. However, some studies suggest that ectopic fat stored in liver (as in NAFLD) poses more risk than the fat in other sites.[16] As of now, there is no answer to the pressing question, “Which is the 'real' obesity?.” Without a standard method of defining and quantifying “unhealthy obesity,” accurately identifying cancers that are distinctly and specifically obesity related will remain imprecise.
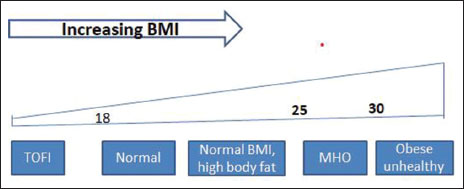
| Figure 2:The spectrum of obesity as per body mass index
Nevertheless, the International Agency for Research on Cancer has come up with a list of 13 cancers associated with obesity[17] (as defined by BMI) [Table 1] including common ones such as those of colon and breast. Since then, other associations have been reported,[6],[18] including new possibilities such as of the prostate,[19] neuroendocrine tumors,[20] and of the urinary bladder.[21] Theoretically, cancers with similar etiology should have similar mutation spectra,[22] but since cancers are rarely caused by a single factor, defining a homogenous population of JRCs and generating a universal “molecular signature” that could identify a cancer as a member of JRC remains difficult. Overexpression of genes such as the fatty acid synthase (FASN)[23] and fat mass and obesity-associated (FTO)[24],[25] have been identified in JRCs.
|
Cancer |
|---|
|
Colon cancer |
|
Breast cancer |
|
Thyroid cancer |
|
Liver cancer |
|
Endometrial cancer |
|
Esophageal cancer |
|
Renal cancer |
|
Gall bladder cancer |
|
Pancreatic cancer |
|
Ovarian cancer |
|
Gastric cardia cancer |
|
Multiple myeloma |
|
Meningioma |
|
Cancer |
Postulated mechanism |
|---|---|
|
NAFLD – Nonalcoholic fatty liver disease |
|
|
Breast cancer (postmenopausal) |
Estrogen produced by adipose tissue |
|
Endometrial cancer |
High estrogen levels |
|
Gastro-esophageal cancer |
Increased gastro-esophageal reflux due to high visceral fat |
|
Gall bladder cancer |
Cholesterol gall stones |
|
Liver cancer |
Fatty liver, NAFLD |
|
Number |
Criterion |
Explanation |
Obesity and cancer |
|---|---|---|---|
|
1 |
Strength |
Difference between exposed versus nonexposed |
Significant for select subsets |
|
2 |
Consistency |
Observed by different people at different places |
Yes |
|
3 |
Specificity |
Linked to specific outcome |
Yes but inconsistent |
|
4 |
Temporality |
Exposure precede the disease |
Yes |
|
5 |
Biological gradient |
Dose response curve |
Yes, with breast cancer |
|
6 |
Plausibility |
Biologically plausible |
Yes |
|
7 |
Coherence |
Cause-effect consistent with known natural history |
Yes but further investigations needed |
|
8 |
Experiment |
Intervention change outcome? |
Yes |
|
9 |
Analogy |
Similar agents cause similar disease? |
Unique experience |

| Figure 1:The carbohydrate-insulin model of obesity

| Figure 2:The spectrum of obesity as per body mass index
References
- rnold M, Leitzmann M, Freisling H, Bray F, Romieu I, Renehan A. et al. Obesity and cancer: An update of the global impact. Cancer Epidemiol 2016; 41: 8-15
- ariosa D, Carreras-Torres R, Martin RM, Johansson M, Brennan P. Commentary: What can Mendelian randomization tell us about causes of cancer?. Int J Epidemiol 2019; 48: 816-21
- rown KF, Rumgay H, Dunlop C, Ryan M, Quartly F, Cox A. et al. The fraction of cancer attributable to modifiable risk factors in England, Wales, Scotland, Northern Ireland, and the United Kingdom in 2015. Br J Cancer 2018; 118: 1130-41
- ong YR, Huo J, Desai R, Cardel M, Deshmukh AA. Deshmukh AA. Excess costs and economic burden of obesity-related cancers in the United States. Value Health 2019; 22: 1378-86
- oroukian SM, Dong W, Berger NA. Changes in age distribution of obesity-associated cancers. JAMA Netw Open 2019; 2: e199261
- jørge T, Häggström C, Ghaderi S, Nagel G, Manjer J, Tretli S. et al. BMI and weight changes and risk of obesity-related cancers: A pooled European cohort study. Int J Epidemiol 2019; 48: 1872-85
- tigler FL, Lustig RH, Ma JI. Mechanisms, pathophysiology, and management of obesity. N Engl J Med 2017; 376: 1491
- udwig DS, Ebbeling CB. The carbohydrate-insulin model of obesity: Beyond “calories in, calories out”. JAMA Intern Med 2018; 178: 1098-103
- oussa O, Arhi C, Ziprin P, Darzi A, Khan O, Purkayastha S. Fate of the metabolically healthy obese-is this term a misnomer? A study from the clinical practice research datalink. Int J Obes (Lond) 2019; 43: 1093-101
- Korduner J, Bachus E, Jujic A, Magnusson M, Nilsson PM. Metabolically healthy obesity (MHO) in the Malmö diet cancer study Epidemiology and prospective risks. Obes Res Clin Pract 2019; 13: 548-54
- Iyengar NM, Arthur R, Manson JE, Chlebowski RT, Kroenke CH, Peterson L. et al. Association of body fat and risk of breast cancer in postmenopausal women with normal body mass index: A secondary analysis of a randomized clinical trial and observational study. JAMA Oncol 2019; 5: 155-63
- Zdrojewicz Z, Popowicz E, Szyca M, Michalik T, Śmieszniak B. TOFI phenotype-its effect on the occurrence of diabetes. Pediatr Endocrinol Diabetes Metab 2017; 23: 96-100
- Shulman GI. Ectopic fat in insulin resistance, dyslipidemia, and cardiometabolic disease. N Engl J Med 2014; 371: 1131-41
- Ross R, Neeland IJ, Yamashita S, Shai I, Seidell J, Magni P. et al. Waist circumference as a vital sign in clinical practice: A Consensus Statement from the IAS and ICCR Working Group on Visceral Obesity. Nat Rev Endocrinol 2020; 16: 177-89
- Woolcott OO, Woolcott RN. Defining cutoffs to diagnose obesity using the relative fat mass (RFM): Association with mortality in NHANES 1999-2014. Int J Obes (Lond) 2020; 44: 1301-10
- Allen AM, Hicks SB, Mara KC, Larson JJ, Therneau TM. The risk of incident extrahepatic cancers is higher in non-alcoholic fatty liver disease than obesity-A longitudinal cohort study. J Hepatol 2019; 71: 1229-36
- Lauby-Secretan B, Scoccianti C, Loomis D, Grosse Y, Bianchini F, Straif K. et al. Body fatness and cancer-viewpoint of the IARC working Group. New England J Med 2016; 375: 794-8
- Carreras-Torres R, Johansson M, Haycock PC, Wade KH, Relton CL, Martin RM. et al. Obesity, metabolic factors and risk of different histological types of lung cancer: A Mendelian randomization study. PLoS One 2017; 12: e0177875
- Genkinger JM, Wu K, Wang M, Albanes D, Black A, van den BrandtPA. et al Measures of body fatness and height in early and mid-to-late adulthood and prostate cancer: Risk and mortality in The pooling project of prospective studies of diet and cancer. Ann Oncol 2020; 31: 103-14
- Santos AP, Santos AC, Castro C, Raposo L, Pereira SS, Torres I. et al. Visceral obesity and metabolic syndrome are associated with well-differentiated gastroenteropancreatic neuroendocrine tumors. Cancer s (Basel) 2018; 10: 293
- Choi JB, Lee EJ, Han KD, Hong SH, Ha US. Estimating the impact of body mass index on bladder cancer risk: Stratification by smoking status. Sci Rep 2018; 8: 947
- Cieslik M, Chinnaiyan AM. Global genomics project unravels cancer's complexity at unprecedented scale. Nature 2020; 578: 39-40
- Wang D, Dubois RN. Associations between obesity and cancer: The role of fatty acid synthase. J Natl Cancer Inst 2012; 104: 343-5
- Akbari ME, Gholamalizadeh M, Doaei S, Mirsafa F. FTO gene affects obesity and breast cancer through similar mechanisms: A new insight into the molecular therapeutic targets. Nutr Cancer 2018; 70: 30-6
- Deng X, Su R, Stanford S, Chen J. Critical enzymatic functions of FTO in obesity and cancer. Front Endocrinol (Lausanne) 2018; 9: 369
- Schünemann H, Hill S, Guyatt G, Akl EA, Ahmed F. The GRADE approach and Bradford Hill's criteria for causation. J Epidemiol Community Health 2011; 65: 392-5
- Campbell PT. Obesity: A certain and avoidable cause of cancer. Lancet 2014; 384: 727-8
- Smith LA, O'Flanagan CH, Bowers LW, Allott EH, Hursting SD. Translating mechanism-based strategies to break the obesity-cancer link: A narrative review. J Acad Nutr Diet 2018; 188: 652-67
- Kompella P, Vasquez KM. Obesity and cancer: A mechanistic overview of metabolic changes in obesity that impact genetic instability. Mol Carcinog 2019; 58: 1531-50
- Erion KA, Corkey BE. Hyperinsulinemia: A cause of obesity?. Curr Obes Rep 2017; 6: 178-86
- Vigneri R, Goldfine ID, Frittitta L. Insulin, insulin receptors, and cancer. J Endocrinol Invest 2016; 39: 1365-76
- Parekh N, Guffanti G, Lin Y, Ochs-Balcom HM, Makarem N, Hayes R. Insulin receptor variants and obesity-related cancers in the Framingham Heart Study. Cancer Causes Control 2015; 26: 1189-95
- Tsujimoto T, Kajio H, Sugiyama T. Association between hyperinsulinemia and increased risk of cancer death in nonobese and obese people: A population-based observational study. Int J Cancer 2017; 141: 102-11
- Turati F, Galeone C, Gandini S, Augustin LS, Jenkins DJ, Pelucchi C. et al. High glycemic index and glycemic load are associated with moderately increased cancer risk. Mol Nutr Food Res 2015; 59: 1384-94
- Nyasani E, Munir I, Perez M, Payne K, Khan S. Linking obesity-induced leptin-signaling pathways to common endocrine-related cancers in women. Endocrine 2019; 63: 3-17
- Yoon YS, Kwon AR, Lee YK, Oh SW. Circulating adipokines and risk of obesity related cancers: A systematic review and meta-analysis. Obes Res Clin Pract 2019; 13: 329-39
- Inagaki-Ohara K. Gastric leptin and tumorigenesis: Beyond obesity. Int J Mol Sci 2019; 20: 2622
- Hao J, Zhang Y, Yan X, Yan F, Sun Y, Zeng J. et al. Circulating adipose fatty acid binding protein is a new link underlying obesity-associated breast/mammary tumor development. Cell Metabe 2018; 28: 689-705
- Starling S. Obesity-linked inflammation tied to glutamine levels. Nat Rev Endocrinol 2020; 16: 130-1
- Quail DF, Dannenberg AJ. The obese adipose tissue microenvironment in cancer development and progression. Nat Rev Endocrinol 2019; 15: 139-54
- Włodarczyk M, Nowicka G. Obesity, DNA damage, and development of obesity-related diseases. Int J Mol Sci 2019; 20: 2622
- Valdes AM, Walter J, Segal E, Spector TD. Role of the gut microbiota in nutrition and health. BMJ 2018; 361: k2179
- Cani PD, Jordan BF. Gut microbiota-mediated inflammation in obesity: A link with gastrointestinal cancer. Nat Rev Gastroenterol Hepatol 2018; 15: 671-82
- Greathouse KL, White JR, Padgett RN, Perrotta BG, Jenkins GD, Chia N. et al. Gut microbiome meta-analysis reveals dysbiosis is independent of body mass index in predicting risk of obesity-associated CRC. BMJ Open Gastroenterol 2019; 6: e000247
- Luo J, Hendryx M, Manson JE, Figueiredo JC, LeBlanc ES, Barrington W. et al. Intentional weight loss and obesity-related cancer risk. JNCI Cancer Spectrum 2019; 3: pkz054
- Zhang K, Luo Y, Dai H, Deng Z. Effects of bariatric surgery on cancer risk: Evidence from meta-analysis. Obesity Surg 2020; 30: 1265-72
- Taube M, Peltonen M, Sjoholm K, Anveden A, Andersson-Assarsson JC, Jacobson P. et al. Association of bariatric surgery with skin cancer incidence in adults with obesity: A nonrandomized controlled trial. JAMA Dermatol 2019; 156: 1-7
- d">48 Iacobucci G. Treat obesity with the same effort used to reduce smoking, says lead psychologist. BMJ 2019; 366: l5713
- Shu X, Wu L, Khankari NK, Shu XO, Wang TJ, Michailidou K. et al. Associations of obesity and circulating insulin and glucose with breast cancer risk: A Mendelian randomization analysis. Int J Epidemiol 2019; 48: 795-806
- Del Pozo MD, Castelló A, Vidal C, Salas-Trejo D, Sánchez-Contador C, Pedraz-Pingarrón C. et al. Overeating, caloric restriction and mammographic density in Spanish women. DDM-Spain study. Maturitas 2018; 117: 57-63
- Moke DJ, Hamilton AS, Chehab L, Deapen D, Freyer DR. Obesity and risk for second malignant neoplasms in childhood cancer survivors: A case-control study utilizing the california cancer registry. Cancer Epidemiol Biomarkers Prev ent 2019; 28: 1612-20
- Heetun A, Cutress RI, Copson ER. Early breast cancer: Why does obesity affect prognosis?. Proc Nutr Soc 2018; 77: 369-81
- Osman MA, Hennessy BTK. Obesity correlation with metastases development and response to first-line metastatic chemotherapy in breast cancer. Clin Med Insights Oncol 2015; 9: 105-12
- Barua R, Templeton AJ, Seruga B, Ocana A, Amir E, Ethier JL. Hyperglycaemia and survival in solid tumours: A systematic review and meta-analysis. Clin Oncol (R Coll Radiol) 2018; 30: 215-24
- Lennon H, Sperrin M, Badrick E, Renehan AG. The obesity paradox in cancer: A review. Curr Oncol Rep 2016; 18: 56
- Renehan AG, Sperrin M. The obesity paradox and mortality after colorectal cancer: A causal conundrum. JAMA Oncol 2016; 2: 1127-9
- Lee DH, Giovannucci EL. The obesity paradox in cancer: Epidemiologic insights and perspectives. Curr Nutr Rep 2019; 8: 175-81
- Sperrin M, Candlish J, Badrick E, Renehan A, Buchan I. Collider bias is only a partial explanation for the obesity paradox. Epidemiology 2016; 27: 525-30
- Ujvari B, Jacqueline C, Misse D, Amar V, Fitzpatrick JC, Jennings G. Obesity paradox in cancer: Is bigger really better?. Evolutionary Appl 2019; 12: 1092-5
- Sanchez A, Furberg H, Kuo F, Vuong L, Ged Y, Patil S. et al. Transcriptomic signatures related to the obesity paradox in patients with clear cell renal cell carcinoma: A cohort study. Lancet Oncol 2020; 21: 283-93
- Renehan AG, Harvie M, Cutress RI, Leitzmann M, Pischon T, Howell S. et al. How to manage the obese patient with cancer. J Clin Oncol 2016; 34: 4284-94
- Lyman GH, Sparreboom A. Chemotherapy dosing in overweight and obese patients with cancer. Nat Rev Clin Oncol 2013; 10: 451-9
- Lehuede C, Li X, Dauvillier S, Vaysse C, Franchet C, Clement E. et al. Adipocytes promote breast cancer resistance to chemotherapy, a process amplified by obesity: Role of the major vault protein (MVP). Breast Cancer Res 2019; 21: 7
- Zhang Z, Scherer PE. Adipose tissue: The dysfunctional adipocyte A cancer cell's best friend. Nat Rev Endocrinol 2018; 14: 132-4
- Kosalka P, Johnson C, Turek M, Sulpher J, Law A, Botros J, Sarin YK. et al. Effect of obesity, dyslipidemia, and diabetes on trastuzumab-related cardiotoxicity in breast cancer. Curr Oncol 2019; 26: e314-e321
- Cespedes Feliciano EM, Chen WY, Lee V, Albers KB, Prado CM, Alexeeff S. et al. Body composition, adherence to anthracycline and taxane-based chemotherapy, and survival after nonmetastatic breast cancer. JAMA Oncol 2019; 6: 264-70
- Lysaght J. The 'obesity paradox' in action with cancer immunotherapy. Nat Rev Endocrinol 2019; 15: 132-3
- d">68 Menendez JA, Lupu R. Fatty acid synthase (FASN) as a therapeutic target in breast cancer. Expert Opin Ther Targets 2017; 21: 1001-16
- McMurray F, Demetriades M, Aik W, Merkestein M, Kramer H, Andrew DS. et al. Pharmacological inhibition of FTO. PLoS One 2015; 10: e0121829
- Prado CM, Lieffers JR, McCargar LJ, Reiman T, Sawyer MB, Martin L. et al. Prevalence and clinical implications of sarcopenic obesity in patients with solid tumours of the respiratory and gastrointestinal tracts: A population-based study. Lancet Oncol 2008; 9: 629-35
- Yip C, Dinkel C, Mahajan A, Siddique M, Cook GJ, Goh V. Imaging body composition in cancer patients: Visceral obesity, sarcopenia and sarcopenic obesity may impact on clinical outcome. Insights Imaging 2015; 6: 489-97
- Ligibel JA, Alfano CM, Courneya KS, Demark-Wahnefried W, Burger RA, Chlebowski RT. et al. American Society of Clinical Oncology position statement on obesity and cancer. J Clin Oncol 2014; 32: 3568-74
- Renehan AG, Lloyd K, Renehan I. Awareness of the link between obesity and cancer in UK school curricula. Lancet 2019; 393: 1591-2
- Venniyoor A. The most important questions in cancer research and clinical oncology-Question 2-5. Obesity-related cancers: More questions than answers. Chin J Cancer 2017; 36: 18
- Misra A, Chowbey P, Makkar BM, Vikram NK, Wasir JS, Chadha D. et al. Consensus statement for diagnosis of obesity, abdominal obesity and the metabolic syndrome for Asian Indians and recommendations for physical activity, medical and surgical management. J Assoc Physicians India 2009; 57: 163-70


 PDF
PDF  Views
Views  Share
Share

