Outcomes and Management of Head and Neck Cancer at a South Indian Cancer Centre: A Retrospective Study
CC BY 4.0 · Indian J Med Paediatr Oncol 2022; 43(06): 500-506
DOI: DOI: 10.1055/s-0042-1758541
Abstract
Introduction Head and neck cancers are one of the most common cancers in the Indian subcontinent. The trends of these cancers worldwide have drastically changed over the past 15 years. In spite of all the new technology and timely diagnosis, the treatment of these cancers is still a challenge. These cancers still continue to be a significant cause of morbidity and mortality worldwide.
Objectives To identify different patterns of care received by patients with primary head and neck cancer in a single center and analyze the outcomes of the different patterns of care received by these patients in terms of overall survival and disease-free survival.
Materials and Methods We included 707 patients with primary head and neck cancer registered and treated in our institution from January 2015 to December 2017. The demographic details of the patient, treatment received, and outcomes of treatment were collected retrospectively from our hospital's medical registry. Descriptive analysis was performed by calculating mean and standard deviation for quantitative variables, whereas frequency and proportion were calculated for categorical variables. The mean/median overall survival and recurrence-free survival were compared across various explanatory parameters using log rank–test. A p-value < 0.05 was considered statistically significant.
Results A total of 707 patients were included in the final analysis. The median age of presentation was 60 years. In total, 50% of patients presented with stage IV disease at diagnosis and 78% had a history of smoking or other tobacco use. Oral cavity was the most common primary site. Concurrent chemotherapy with radiation therapy was the most common modality of treatment used in 49% of patients: RT was the common modality of treatment in 21% patients. Fourteen percent patients were treated by only surgery. All patients who underwent treatment were included for survival analysis, which showed that the median overall survival time was 42 months (34–49 months). The median duration of disease free-survival time was 37 months (30–43 months).
Conclusion In our study, most patients presented with locally advanced disease. Multimodality treatment yielded better results. Based on our study, in early-stage cancer, where single modality treatment was used, adjuvant therapy should be tailored based on nomogram.
Keywords
head and neck cancer - survival - epidemiology - patterns of care - outcome - radiotherapy - chemotherapyPublication History
Article published online:
05 December 2022
© 2022. The Author(s). This is an open access article published by Thieme under the terms of the Creative Commons Attribution License, permitting unrestricted use, distribution, and reproduction so long as the original work is properly cited. (https://creativecommons.org/licenses/by/4.0/)
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India
Abstract
Introduction Head and neck cancers are one of the most common cancers in the Indian subcontinent. The trends of these cancers worldwide have drastically changed over the past 15 years. In spite of all the new technology and timely diagnosis, the treatment of these cancers is still a challenge. These cancers still continue to be a significant cause of morbidity and mortality worldwide.
Objectives To identify different patterns of care received by patients with primary head and neck cancer in a single center and analyze the outcomes of the different patterns of care received by these patients in terms of overall survival and disease-free survival.
Materials and Methods We included 707 patients with primary head and neck cancer registered and treated in our institution from January 2015 to December 2017. The demographic details of the patient, treatment received, and outcomes of treatment were collected retrospectively from our hospital's medical registry. Descriptive analysis was performed by calculating mean and standard deviation for quantitative variables, whereas frequency and proportion were calculated for categorical variables. The mean/median overall survival and recurrence-free survival were compared across various explanatory parameters using log rank–test. A p-value < 0.05 was considered statistically significant.
Results A total of 707 patients were included in the final analysis. The median age of presentation was 60 years. In total, 50% of patients presented with stage IV disease at diagnosis and 78% had a history of smoking or other tobacco use. Oral cavity was the most common primary site. Concurrent chemotherapy with radiation therapy was the most common modality of treatment used in 49% of patients: RT was the common modality of treatment in 21% patients. Fourteen percent patients were treated by only surgery. All patients who underwent treatment were included for survival analysis, which showed that the median overall survival time was 42 months (34–49 months). The median duration of disease free-survival time was 37 months (30–43 months).
Conclusion In our study, most patients presented with locally advanced disease. Multimodality treatment yielded better results. Based on our study, in early-stage cancer, where single modality treatment was used, adjuvant therapy should be tailored based on nomogram.
Keywords
head and neck cancer - survival - epidemiology - patterns of care - outcome - radiotherapy - chemotherapyIntroduction
Head and neck cancers (HNCs) are a heterogenous group of cancers that arise from the mucosa of the aerodigestive tract. According to the GLOBOCAN 2018 data, 2,05,325 new cases of HNC are diagnosed in a year and 1,22,834 deaths are associated with HNC in India. Cancers of the lip and oral cavity are the second most common cancers following breast cancer. HNC constitutes 10.4% of the cancer burden and they account for 16.1% of cases in males and 4.8% of the cases in females.[1] Tobacco exposure and alcohol dependence are the two main causes of HNCs.[2] Over the past 15 years, the trends have drastically changed with increased incidence of human papilloma virus causing HNCs.[3] [4] The majority of HNCs are diagnosed at late stages.[5] Inspite of changes in technology, the diagnosis and management of these tumors are still a challenge. The aim of this study was to describe the modalities of treatment and outcomes in patients with head and neck cancer.
Materials and Methods
This study was a retrospective observational study.
Inclusion Criteria
All newly diagnosed primary head and neck cancer belonging to these sites–oral cavity, oropharynx, hypopharynx, nasopharynx, and larynx between January 2015 and December 2017 were included in the analysis. Cases with primary head and cancer treated during the study period and who developed recurrence or progression during follow-up were recorded. In the cases showing advanced stage of the diseases, treatment received in form of palliative radiotherapy, palliative chemotherapy was also included.
Exclusion Criteria
Those patients who had treatment elsewhere were excluded from the study.
The demographic details of the study population, tumor characteristics, stage (according to American Joint committee on cancer 7th edition), and treatment received were collected from the hospital's medical registry records. All patients were discussed in the institutional Tumor Board meeting (Departments involved were Radiation oncology, Medical Oncology Surgical Oncology, Palliative Care, Pathology and Radiology) and the treatment was decided. Patients treated during the study period and developed recurrence or progression during follow-up were recorded. Primary outcome of the treatment was assessed after 2 months following the completion of treatment with history, clinical examination, imaging (CT scan neck with contrast) and was defined in terms of complete response (CR), partial response (PR), residual disease or static disease. This was evaluated according to Response Evaluation Criteria in Solid Tumors (RECIST) criteria version 1.0. The overall outcome of treatment is defined in terms of overall survival (OS) and disease-free survival (DFS). Overall survival is defined as the time from the date of diagnosis of the disease to the date of death of the patient due to any cause. Disease-free survival is defined as the time from the date of completion of treatment to the date of detection of recurrence. The follow-up strategy was for the first year, every 3 months, for the second year every 6 months, and for the third year every 12 months. These follow-ups included assessment of medical history, physical examination (complete head and neck exam; mirror and fiberoptic examination as clinically indicated), imaging (CT scan neck/MRI) done 8 weeks after completion of treatment and then yearly if the patient is symptomatic, and chest X-ray.
Statistical methods: Sample size was around 400 patients calculated using formula n = t2 × p(1 − p)/m2
Descriptive analysis was performed by evaluating mean and standard deviation for quantitative variables, whereas frequency and proportion were evaluated for categorical variables. Key outcomes included overall survival and disease-free survival. The key explanatory parameters considered for the analysis were the demographic characteristics of the patient such as age, past history of co-morbidities, disease characteristics, and treatment-related parameters. If the data were not available on any particular explanatory parameter they were considered as missing values and were excluded from the analysis while assessing the association of that factor with disease-free survival. The number of proportion of the missing values for each parameter was explicitly mentioned in the descriptive analysis. For survival analysis cases, data lost to follow-up or missing data were censored. Differences between groups in the median overall survival and disease-free survival according to possible explanatory parameters were assessed with log-rank test, and presented with Kaplan–Meier survival plots. A p-value < 0.05 was considered statistically significant. The IBM SPSS version 22 software was used for statistical analysis (IBM Corp.Released 2013. IBM SPSS statistics for windows, Version 22.Armonk, NY:IBM Corp).
Ethics
All procedures followed were in accordance with ethical standards of the responsible committee on human experimentation (institutional or regional) and with the Helsinki Declaration of 1975, as revised in 2013.The informed consent for this study was waived by the institutional ethics committee, G. Kuppuswamy Naidu Memorial Hospital, Coimbatore, Tamil Nadu as this is a retrospective study. The code for this study was (ECR209/INS/TN2013/RR-19 .dated 28 December 2019).
Results
We included a total of 707 subjects. During the study period of 3 years, the incidence of primary HNC was around 250 per year (35%).
Patient Characteristics
The age of diagnosis ranged from 27 year to 92 years with a median age being 60 years. There was a 1-year-old child diagnosed with rhabdomyosarcoma of the posterior tongue. The disease was more common in men than women (77% versus 22%) with a ratio of 3:1. Over 90% had an ECOG performance status 1 ([Table 1]).
|
Parameters |
Percentage |
|---|---|
|
Age group |
|
|
Up to 50 |
20.79% (147) |
|
51 to 70 |
59.83% (423) |
|
> 70 |
19.38% (137) |
|
Smoking/tobacco history |
|
|
Yes |
77.51% (548) |
|
Alcohol history |
|
|
Yes |
49.50% (350) |
|
Site |
|
|
Oral cavity |
40.45% (286) |
|
Oropharynx |
23.62% (167) |
|
Hypopharynx |
19.94% (141) |
|
Larynx |
11.46% (81) |
|
Others |
4.52% (32) |
|
Stage grouping |
|
|
I |
12.73% (90) |
|
II |
13.72% (97) |
|
III |
18.81% (133) |
|
IVA |
50.50% (357) |
|
IVB |
4.24% (30) |
Treatment planned |
Frequency |
Percentages |
|---|---|---|
|
RT + CT |
339 |
47.95 |
|
RT |
151 |
21.36 |
|
Surgery |
100 |
14.14 |
|
Surgery→ Adjuvant RT ± CT |
74 |
10.47 |
|
NACT→ RT ± CT |
13 |
1.84 |
|
Others |
30 |
4.24 |
|
Treatment planned |
Frequency |
Percentages |
|---|---|---|
|
RT + CT |
198 |
49 |
|
RT |
85 |
21 |
|
Surgery |
58 |
14 |
|
Surgery→ Adjuvant RT ± CT |
48 |
12 |
|
NACT→ RT ± CT |
4 |
1 |
|
Others |
12 |
3 |
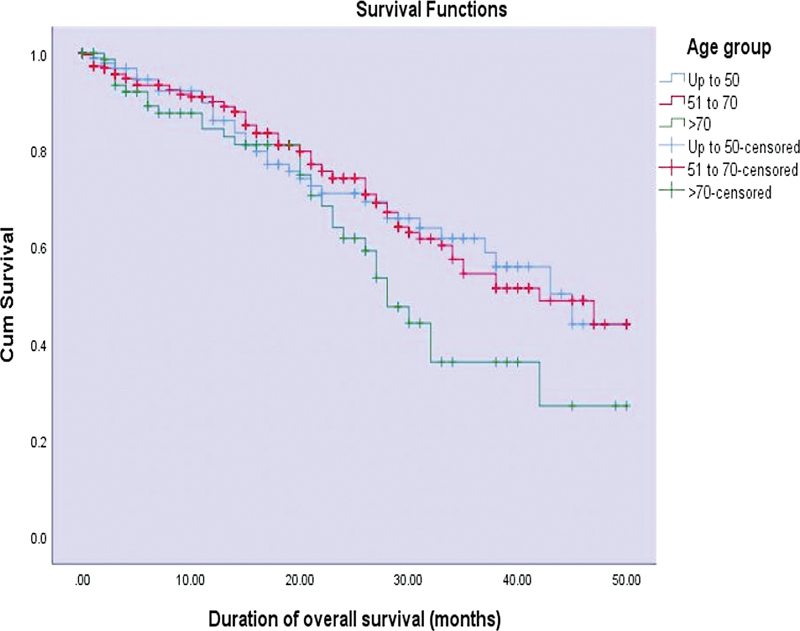
| Figure 1:Kaplan–Meier curves of duration of overall survival (months) across various age groups (p-value = 0.100).
The median overall survival at the time of diagnosis was found to be 42 months in patients who had stage I and II disease, 45 months in stage III disease, and 37 months in stage IV disease. Similarly, the median DFS was evaluated to be 42 months in early stage I and II disease, 30 months in stage III disease, and 35 months in stage IV disease. Cancers of the hypopharynx and larynx had a median survival of 45 months, followed by 43 months for oral cavity, 33 months for nasopharynx, and unknown primary tumor with neck as the secondary site: 31 months for oropharynx and 28 months for malignancies involving paranasal sinus, ear and nasal cavity ([Fig. 2]). Similarly, hypopharynx and larynx had median DFS of 42 months followed by 38 months in the oral cavity, 33 months in the nasopharynx, and unknown primary tumor; and 26 months in the oropharynx. Patients treated with CRT had a median survival period of 38 months. Treatment with CRT was associated with a DFS of 31 months compared with other combined modalities, which had a DFS of 33 months.
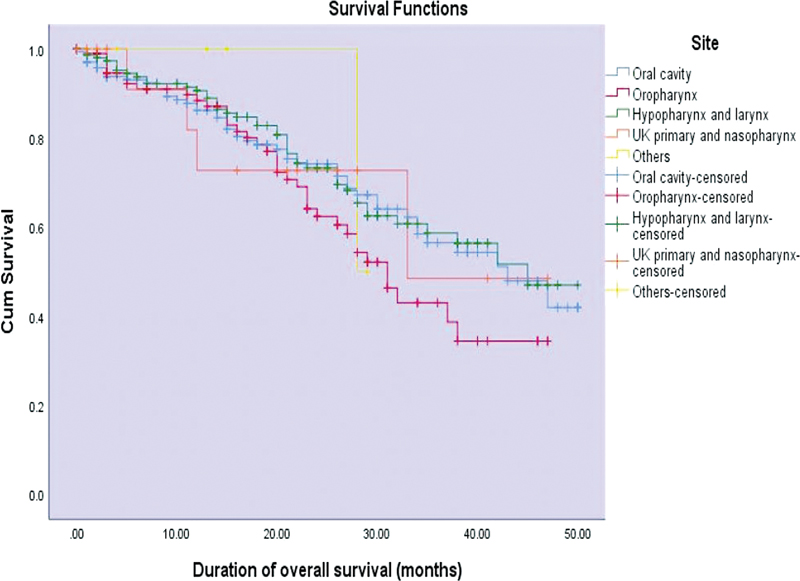
| Figure 2:Kaplan–Meier curves of duration of overall survival (months) across various sites of head and neck (p-value = 0.100).
Discussion
Our institution data on patients diagnosed with head and neck cancer between January 2015 to December 2017 showed that the disease was more common among men with a median age of diagnosis of 60 years. Around 54.7% of patients presented with a stage IV disease at the time of diagnosis. Association with history of smoking or any kind of tobacco use was found in 77.5% of patients in the study. The strong association between tobacco and several HNC has been well established by Wynder et al and several other studies in the literature.[6] [7]
A report from south Indian population shows that the trend is emerging showing that there is a definite increase in the number of patients presenting with tongue cancers.[8]
Oral cavity was the most common site involved followed by oropharynx and hypopharynx. Concurrent chemoradiation was the most common modality of treatment used. All patients who underwent treatment were included for survival analysis which showed that median overall survival was 42 months and the median DFS was 37 months. Decreased survival was found in patients more than 70 years of age, with cancers of the oropharynx and having stage IV disease.
This study was a retrospective observational study in which data were collected from a hospital-based cancer registry. Cancer registries are good sources of information on the demographics, tumor characteristics, and stage at diagnosis.[9] Benefit of any treatment in oncology can be defined in two ways-the patient either live longer (OS) or live better (q uality of life). OS is a universally accepted measure of benefit and is the most commonly used measure.[10] Because we wanted to compare the outcomes of the different patterns of treatment in our institute, overall survival was considered as the primary end point. Disease-free survival is a surrogate end point and is most commonly used to assess the benefit of adjuvant treatment.
However, with improvements in modern radiation techniques, the intent of treatment in HNC has been more inclined toward cure with functional preservation. This could explain why CRT was the major modality of treatment preferred for locally advanced HNC in our study population. More than 50% of the study population presented with stage IV disease at the time of diagnosis. Similar presentations were found in the retrospective study performed by Roy et al.[11] The outcomes and epidemiology of HNC were evaluated from a cancer registry, and it was found that 49% of the study population had stage IV disease at the time of presentation.[12] This can be attributed to illiteracy and lack of awareness among the general population regarding the disease. In developing countries such as India, the majority of the people presented with advanced disease at the time of diagnosis. Therefore, HNC in developing countries contribute to a significant mortality and morbidity.[13]
In our study, the primary response was assessed 2 months after completion of treatment in 405 patients. It showed that 69.3% of patients had a complete response to treatment;22.2% had residual disease, 2.7% had a progressive disease, and 5.6% were lost to follow-up. Steinbichler et al in Austria performed a study on persistent disease following first line treatment in HNC. Out of the 741 patients studied, 76% had complete response to treatment, 24% had persistent or residual disease.[14]
Out of the 707 patients, survival analysis was performed for 463 patients who underwent treatment at our center. The median overall survival was evaluated to be 42 months and the median disease-free survival time was evaluated to be 37 months. The median overall survival and disease-free survival were found to be decreased in patients of age > 70 years (OS-28 months, DFS-27 months) when compared with those < 50 years (OS-45 months, DFS-43 months). However, this difference was not found to be statistically significant.
Both median OS and DFS were found to be decreased in cancers of oropharynx when compared with cancers of the oral cavity, hypopharynx, and larynx. In our study, laryngeal cancers are found to have better survival when compared with other sites. These results were in accordance with study by Cadoni et al and Kambiz et al, where the median survival time was higher for laryngeal cancers and reduced survival was associated with increasing age of diagnosis and advanced tumor stage.[15] [16] In both of these studies, the 4-year overall survival for all HNC sites was around 60%. Cancers involving nasal cavities, paranasal sinuses, and ears had very poor survival rate. HPV positivity was more commonly found in oropharyngeal cancers among non-smokers.[17] [18] HPV 16 positivity was associated with an increased risk of HNC, especially oropharyngeal cancers.[19] Future studies should include HPV data to observe its impact on the multimodality treatment.
In our center, single modality of treatment was recommended for early-stage localized diseases. Multimodality of treatment including surgery, chemotherapy, and radiation therapy had a slightly better survival than CRT. However, this difference was not statistically significant.
Accelerated radiation and hypofractionation are effective methods of increasing therapeutic benefits of radiation. A meta-analysis on the role of chemotherapy in HNC (MACH-NC) showed that hypofractionated RT with concurrent chemotherapy was the best modality.[20]
Latest update of (MACH-NC) is that overall survival was not increased by addition of induction or adjuvant chemotherapy. Efficacy of induction chemotherapy decreases with poorer performance status.
It has been shown that hypofractionated RT can achieve similar tumor response to conventional fractionated RT in HNC although with some increased toxicity.[21]
·Similarly, randomized RTOG trials showed hyperfractionated RT had better local control and overall survival compared with conventional fractionation in HNC.[22] [23]
The strength of the study was the number of patients, data being collected from a hospital-based cancer registry, treatment of all patients being decided by a multidisciplinary team of medical professionals.
Limitations
The maximum duration of follow-up observed in our study was only 50 months and the last patient recruited was in December 2017. Due to limitations in time and resources, a 3-year or 5-year survival analysis could not be performed. This would have given us more scientific and meaningful conclusions on the outcomes of HNC.
In this study, we have not assessed the HPV status. Recently, we started testing HPV status for all oropharyngeal cancers for its future potential implications. We are also planning for clinical trials including dose escalation strategies in locally advanced head and neck cancer particularly in non-responders.
Conclusion
In our study, most patients presented with locally advanced disease. Multimodality treatment yields better results. Based on our study in early-stage cancer where single modality treatment was used, adjuvant therapy should be tailored based on nomogram.
Conflict of Interest
None declared.
Acknowledgment
Martin Stockler, Professor of Oncology and Clinical Epidemiology University of Sydney for his valuable suggestions such as manuscript editing and review.
References
- Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018; 68 (06) 394-424
- Rettig EM, D'Souza G. Epidemiology of head and neck cancer. Surg Oncol Clin N Am 2015; 24 (03) 379-396
- Johnson-Obaseki S, McDonald JT, Corsten M, Rourke R. Head and neck cancer in Canada: trends 1992 to 2007. Otolaryngol Head Neck Surg 2012; 147 (01) 74-78
- Rezende TMB, de Souza Freire M, Franco OL. Head and neck cancer: proteomic advances and biomarker achievements. Cancer 2010; 116 (21) 4914-4925
- Mehanna H, Paleri V, West CM, Nutting C. Head and neck cancer–Part 1: Epidemiology, presentation, and prevention. BMJ 2010; 341 (7774): c4684
- Wynder EL, Bross IJ, Feldman RM. A study of the etiological factors in cancer of the mouth. Cancer 1957; 10 (06) 1300-1323
- Franceschi S, Levi F, La Vecchia C. et al. Comparison of the effect of smoking and alcohol drinking between oral and pharyngeal cancer. Int J Cancer 1999; 83 (01) 1-4
- Francis D. Trends in the incidence of head and neck cancers in India. Eur J Cancer India 2018; 92 (01) S23
- nbsp;Warnakulasuriya S. Global epidemiology of oral and oropharyngeal cancer. Oral Oncol 2009; 45 (4-5): 309-316
- Guntinas-Lichius O, Wendt TG, Kornetzky N. et al. Trends in epidemiology and treatment and outcome for head and neck cancer: a population-based long-term analysis from 1996 to 2011 of the Thuringian cancer registry. Oral Oncol 2014; 50 (12) 1157-1164
- Roy S, Mandal TK, Das S. et al. Demography and pattern of care of patients with head-and-neck carcinoma: Experience from a tertiary care center in North India. Cancer Res Stat Treat 2020; 3: 730-735
- Das R, Kataki AC, Sharma JD, Baishya N, Kalita M, Krishnatreya M. A study of head and neck cancer patients with special reference to tobacco use and educational level. Clin Cancer Investig J 2017; 6 (01) 21
- Kulkarni MR. Head and neck cancer burden in India. Int J Head Neck Surg 2013; 4 (01) 29-35
- Steinbichler TB, Lichtenecker M, Anegg M. et al. Persistent head and neck cancer following first-line treatment. Cancers (Basel) 2018; 10 (11) 421
- Cadoni G, Giraldi L, Petrelli L. et al. Prognostic factors in head and neck cancer: a 10-year retrospective analysis in a single-institution in Italy. Acta Otorhinolaryngol Ital 2017; 37 (06) 458-466
- Novin K, Ameri A, Faraji S, Torbati P, Mortazavi N. Head and neck squamous cell carcinoma in Iran: Clinico-pathological and treatment-related factors influencing survival. Iran J Cancer Prev 2015; 8 (05) e3842
- Fouret P, Monceaux G, Temam S, Lacourreye L, St Guily JL. Human papillomavirus in head and neck squamous cell carcinomas in nonsmokers. Arch Otolaryngol Head Neck Surg 1997; 123 (05) 513-516
- Gillison ML, Koch WM, Capone RB. et al. Evidence for a causal association between human papillomavirus and a subset of head and neck cancers. J Natl Cancer Inst 2000; 92 (09) 709-720
- Mork J, Lie AK, Glattre E. et al. Human papillomavirus infection as a risk factor for squamous-cell carcinoma of the head and neck. N Engl J Med 2001; 344 (15) 1125-1131
- Blanchard P, Landais C, Lacas B, Petit C, Bourhis J, Pignon JP. SP-010: update of the meta-analysis of chemotherapy in head and neck cancer (MACH-NC). Radiother Oncol 2017; 1 (122) 9
- Roy S, Mallik C, Ghorai S, Hazra A, Majumdar A. Hypofractionated versus conventional radiotherapy with or without chemotherapy in head and neck cancer: a comparative study. Clin Cancer Investig J 2015; 4: 140-146
- Beitler JJ, Zhang Q, Fu KK. et al. Final results of local-regional control and late toxicity of RTOG 9003: a randomized trial of altered fractionation radiation for locally advanced head and neck cancer. Int J Radiat Oncol Biol Phys 2014; 89 (01) 13-20
- Trotti III A, Zhang Q, Bentzen SM. et al. Randomized trial of hyperfractionation versus conventional fractionation in T2 squamous cell carcinoma of the vocal cord (RTOG 9512). Int J Radiat Oncol Biol Phys 2014; 89 (05) 958-963
Address for correspondence
Publication History
Article published online:
05 December 2022
© 2022. The Author(s). This is an open access article published by Thieme under the terms of the Creative Commons Attribution License, permitting unrestricted use, distribution, and reproduction so long as the original work is properly cited. (https://creativecommons.org/licenses/by/4.0/)
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India

| Figure 1:Kaplan–Meier curves of duration of overall survival (months) across various age groups (p-value = 0.100).

| Figure 2:Kaplan–Meier curves of duration of overall survival (months) across various sites of head and neck (p-value = 0.100).
References
- Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018; 68 (06) 394-424
- Rettig EM, D'Souza G. Epidemiology of head and neck cancer. Surg Oncol Clin N Am 2015; 24 (03) 379-396
- Johnson-Obaseki S, McDonald JT, Corsten M, Rourke R. Head and neck cancer in Canada: trends 1992 to 2007. Otolaryngol Head Neck Surg 2012; 147 (01) 74-78
- Rezende TMB, de Souza Freire M, Franco OL. Head and neck cancer: proteomic advances and biomarker achievements. Cancer 2010; 116 (21) 4914-4925
- Mehanna H, Paleri V, West CM, Nutting C. Head and neck cancer–Part 1: Epidemiology, presentation, and prevention. BMJ 2010; 341 (7774): c4684
- Wynder EL, Bross IJ, Feldman RM. A study of the etiological factors in cancer of the mouth. Cancer 1957; 10 (06) 1300-1323
- Franceschi S, Levi F, La Vecchia C. et al. Comparison of the effect of smoking and alcohol drinking between oral and pharyngeal cancer. Int J Cancer 1999; 83 (01) 1-4
- Francis D. Trends in the incidence of head and neck cancers in India. Eur J Cancer India 2018; 92 (01) S23
- nbsp;Warnakulasuriya S. Global epidemiology of oral and oropharyngeal cancer. Oral Oncol 2009; 45 (4-5): 309-316
- Guntinas-Lichius O, Wendt TG, Kornetzky N. et al. Trends in epidemiology and treatment and outcome for head and neck cancer: a population-based long-term analysis from 1996 to 2011 of the Thuringian cancer registry. Oral Oncol 2014; 50 (12) 1157-1164
- Roy S, Mandal TK, Das S. et al. Demography and pattern of care of patients with head-and-neck carcinoma: Experience from a tertiary care center in North India. Cancer Res Stat Treat 2020; 3: 730-735
- Das R, Kataki AC, Sharma JD, Baishya N, Kalita M, Krishnatreya M. A study of head and neck cancer patients with special reference to tobacco use and educational level. Clin Cancer Investig J 2017; 6 (01) 21
- Kulkarni MR. Head and neck cancer burden in India. Int J Head Neck Surg 2013; 4 (01) 29-35
- Steinbichler TB, Lichtenecker M, Anegg M. et al. Persistent head and neck cancer following first-line treatment. Cancers (Basel) 2018; 10 (11) 421
- Cadoni G, Giraldi L, Petrelli L. et al. Prognostic factors in head and neck cancer: a 10-year retrospective analysis in a single-institution in Italy. Acta Otorhinolaryngol Ital 2017; 37 (06) 458-466
- Novin K, Ameri A, Faraji S, Torbati P, Mortazavi N. Head and neck squamous cell carcinoma in Iran: Clinico-pathological and treatment-related factors influencing survival. Iran J Cancer Prev 2015; 8 (05) e3842
- Fouret P, Monceaux G, Temam S, Lacourreye L, St Guily JL. Human papillomavirus in head and neck squamous cell carcinomas in nonsmokers. Arch Otolaryngol Head Neck Surg 1997; 123 (05) 513-516
- Gillison ML, Koch WM, Capone RB. et al. Evidence for a causal association between human papillomavirus and a subset of head and neck cancers. J Natl Cancer Inst 2000; 92 (09) 709-720
- Mork J, Lie AK, Glattre E. et al. Human papillomavirus infection as a risk factor for squamous-cell carcinoma of the head and neck. N Engl J Med 2001; 344 (15) 1125-1131
- Blanchard P, Landais C, Lacas B, Petit C, Bourhis J, Pignon JP. SP-010: update of the meta-analysis of chemotherapy in head and neck cancer (MACH-NC). Radiother Oncol 2017; 1 (122) 9
- Roy S, Mallik C, Ghorai S, Hazra A, Majumdar A. Hypofractionated versus conventional radiotherapy with or without chemotherapy in head and neck cancer: a comparative study. Clin Cancer Investig J 2015; 4: 140-146
- Beitler JJ, Zhang Q, Fu KK. et al. Final results of local-regional control and late toxicity of RTOG 9003: a randomized trial of altered fractionation radiation for locally advanced head and neck cancer. Int J Radiat Oncol Biol Phys 2014; 89 (01) 13-20
- Trotti III A, Zhang Q, Bentzen SM. et al. Randomized trial of hyperfractionation versus conventional fractionation in T2 squamous cell carcinoma of the vocal cord (RTOG 9512). Int J Radiat Oncol Biol Phys 2014; 89 (05) 958-963


 PDF
PDF  Views
Views  Share
Share

