Palliative chemotherapy in head and neck squamous cell cancer - What is best in Indian population? A time without symptoms, treatment toxicity score based study
CC BY-NC-ND 4.0 · Indian J Med Paediatr Oncol 2013; 34(01): 11-15
DOI: DOI: 10.4103/0971-5851.113404
Abstract
Background: Patients with recurrent and metastatic head and neck Squamous Cell Cancer (HNSCC) have poor prognosis with limited treatment options. In view of decimal prognosis, the treatment decision should include quality of life (QOL) issues, cost-effectiveness besides the response rates and survival. Aim: Present retrospective analysis was conducted to evaluate efficacy (disease-free survival), pharmacoeconomics, and toxicity profile of four (4) different regimens, viz. gefitinib alone, gefitinib with methotrexate, methotrexate alone, or 5-FU with cisplatin. Materials and Methods: Case records between 2007 September and 2008 September were analyzed, 68 patients were found suitable for analysis. Patients received gefitinib (250 mg/day), methotrexate as 50 mg intramuscular weekly or a combination of the same or 5-FU 750 mg/m 2 /day for 4 days along with cisplatin 75 mg/m 2 /day on day 1 in 21-day cycle. Results: A total of 68 patients received therapy. Fifty-one patients have clinically meaningful response (stable disease + complete + partial responses) (75%) and had symptomatic improvement. The median progression-free survival was significantly superior in responders (those who achieved partial or complete response) (8.4 months vs. 3.1 months, P=0.001). Methotrexate with gefitinib had maximum median survival and better overall QOL compared to the other treatment regimens. Weekly methotrexate is relatively cost-effective followed by methotrexate with gefitinib and gefitinib alone. 5-FU with cisplatin in our experience does not appear so attractive in view of high complication rates (when given in full doses) and prolonged hospital stay. Conclusion: Based on the results of this retrospective analysis, methotrexate weekly as single agent or in combination with gefitinib appears as an attractive alternative regimen for patients with metastatic HNSCC including those having poor performance status. A prospective study was planned and submitted to the local ethics committee based on above results to validate these results and compare methotrexate and gefitinib arm with 5-FU + cisplatin.
Publication History
Article published online:
20 July 2021
© 2013. Indian Society of Medical and Paediatric Oncology. This is an open access article published by Thieme under the terms of the Creative Commons Attribution-NonDerivative-NonCommercial-License, permitting copying and reproduction so long as the original work is given appropriate credit. Contents may not be used for commercial purposes, or adapted, remixed, transformed or built upon. (https://creativecommons.org/licenses/by-nc-nd/4.0/.)
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India
Abstract
Background:
Patients with recurrent and metastatic head and neck Squamous Cell Cancer (HNSCC) have poor prognosis with limited treatment options. In view of decimal prognosis, the treatment decision should include quality of life (QOL) issues, cost-effectiveness besides the response rates and survival.
Aim:
Present retrospective analysis was conducted to evaluate efficacy (disease-free survival), pharmacoeconomics, and toxicity profile of four (4) different regimens, viz. gefitinib alone, gefitinib with methotrexate, methotrexate alone, or 5-FU with cisplatin.
Materials and Methods:
Case records between 2007 September and 2008 September were analyzed, 68 patients were found suitable for analysis. Patients received gefitinib (250 mg/day), methotrexate as 50 mg intramuscular weekly or a combination of the same or 5-FU 750 mg/m2/day for 4 days along with cisplatin 75 mg/m2/day on day 1 in 21-day cycle.
Results:
A total of 68 patients received therapy. Fifty-one patients have clinically meaningful response (stable disease + complete + partial responses) (75%) and had symptomatic improvement. The median progression-free survival was significantly superior in responders (those who achieved partial or complete response) (8.4 months vs. 3.1 months, P=0.001). Methotrexate with gefitinib had maximum median survival and better overall QOL compared to the other treatment regimens. Weekly methotrexate is relatively cost-effective followed by methotrexate with gefitinib and gefitinib alone. 5-FU with cisplatin in our experience does not appear so attractive in view of high complication rates (when given in full doses) and prolonged hospital stay.
Conclusion:
Based on the results of this retrospective analysis, methotrexate weekly as single agent or in combination with gefitinib appears as an attractive alternative regimen for patients with metastatic HNSCC including those having poor performance status. A prospective study was planned and submitted to the local ethics committee based on above results to validate these results and compare methotrexate and gefitinib arm with 5-FU + cisplatin.
INTRODUCTION
In India, head and neck cancer is one of the leading cancers (21%).[1] in males (even more than lung cancer, if all subsites are clubbed together) and one of the important cancers in females as well. Its incidence in India is much higher than the rest of world (7%), mostly due to the tobacco chewing habit and other cultural factors like chutta. Most of the cases present in locally advanced stage and often recur either locally or at distant sites despite receiving adequate treatment. The recent meta-analysis suggested that methotrexate single agent appears to be a good choice, though many other regimens had equal or slightly better response rates (range: 15-40%, overall response rates), in view of ease of administration and lesser hospital visits.[2,3] However, the outcome to treatment appears disappointing in view of short progression-free survival (PFS) as well as overall survival. With approximately 90% of (HNSCC) tumors expressing epidermal growth factor receptor (EGFR), EGFR targeting appears to be an attractive target.[4] In view of ease of administration, tyrosine kinase inhibitors (like gefitinib.[4,5,6,7,8,9] or erlotinib) are better than monoclonal antibodies (cetuximab,[10] matuzumab, or panitumumab) in terminally ill patients. Though there is a single study addressing the use of gefitinib from India, quality of life (QOL) and pharmacoeconomic issues were not addressed in it. Therefore, we retrospectively evaluated our data (the QOL questionnaire used in this study was a part of validating the same in various cancers. We used the results of QOL in Head and Neck cancer patients in our analysis) of recurrent and metastatic HNSCC patients receiving one of the four regimens, i.e. gefitinib (250 mg/day), methotrexate as 50 mg intramuscular weekly or a combination of the same or 5-FU 750 mg/m2/day for 4 days along with cisplatin 75 mg/m2/day on day 1 in a 21-day cycle.
MATERIALS AND METHODS
The retrospective analysis was done from the case records of the patients with HNSCC receiving one of the above-mentioned protocols during the period of 2007 September to 2008 September. As this analysis was an afterthought (in another study where the time without symptoms, treatment toxicity (TWISTT) score and QOL questionnaire were being validated in local language across various cancers, we observed that in head and neck cancer, there is significant differences among various patients, whereas in other malignancies, it was not so obvious), all the patients were not treated by the same oncologist and they have varied personal choices. However, to maintain uniformity, we have selected all the patients having the criteria mentioned below for analysis. A total of 68 patients were found eligible for the analysis. The eligibility criteria of patients for analysis include:
- Histologically proven squamous cell carcinoma of the head and neck regions
- Stage IV at the time of initial diagnosis or at recurrence (in case of recurrence, patients must have been adequately treated as per the NCCN guidelines)[11]
- Patients must have at least one measurable lesion as per the Response Evaluation Criteria In Solid Tumors (RECIST) criteria and have follow-up scans done[12]
- Must have completed a minimum of three cycles of therapy
- Patients should not have had any contraindications for the therapy
- Availability of all clinical, pathological, and QOL-related details
All the baseline characters of the patients were recorded from the clinical case records. American Joint Committee on Cancer- tumor node metastasis (AJCC-TNM) system was used for staging the disease. Patients were assessed 3 weekly and Common Toxicity Criteria version 3.0 was used to assess the toxicity.[13] We selected those patients whose dose modifications were done as per the literature requirements. RECIST criteria were used to evaluate the response to therapy and patients were grouped as complete response (CR), no evidence of disease; partial response (PR), more than 30% reduction in sum of the maximum diameter; progressive disease (PD), increase in more than 20% size from the minimum measured disease; and stable disease (SD). Overall, response rate was defined as CR + PR and clinically meaningful response was defined as CR + PR + SD. QOL questionnaire, head and neck (in Telugu) was used to assess the QOL.[14] The cost-effective analysis included the costs involved in the following:
- The systemic therapy (chemo/targeted)
- Management of the complications
- Hospital stay and travel expenses
- Work days lost (calculated for loss of income) in Indian currency.
TWISTT score was calculated by deducting the sum of time spent with complications/worsening of the baseline symptoms, and for treatment from the overall survival of the patients from the initial diagnosis of metastatic head and neck cancer.
Student's t-test was used for the comparison of the various domains in the QOL, response rates, baseline characters, and cost-effective analysis. Intention to treat analysis was used for the assessment of the disease or PFS and the comparison between various regimens was done using the Kaplan Maier curves.
TREATMENT PROTOCOL
- Gefitinib was initially administered orally in a dose of 250 mg once daily either as a single agent or in combination with CT. Usually, patients with poorer performance status received gefitinib as a single agent while the others received CT.
- Methotrexate was given as 50 mg intramuscular weekly with monitoring as mentioned above.
- A combination of gefitinib with methotrexate as mentioned in point 2.
- 5-FU 750 mg/m2/day for 4 days along with cisplatin 75 mg/m2/day on day 1 in a 21-day cycle.
RESULTS
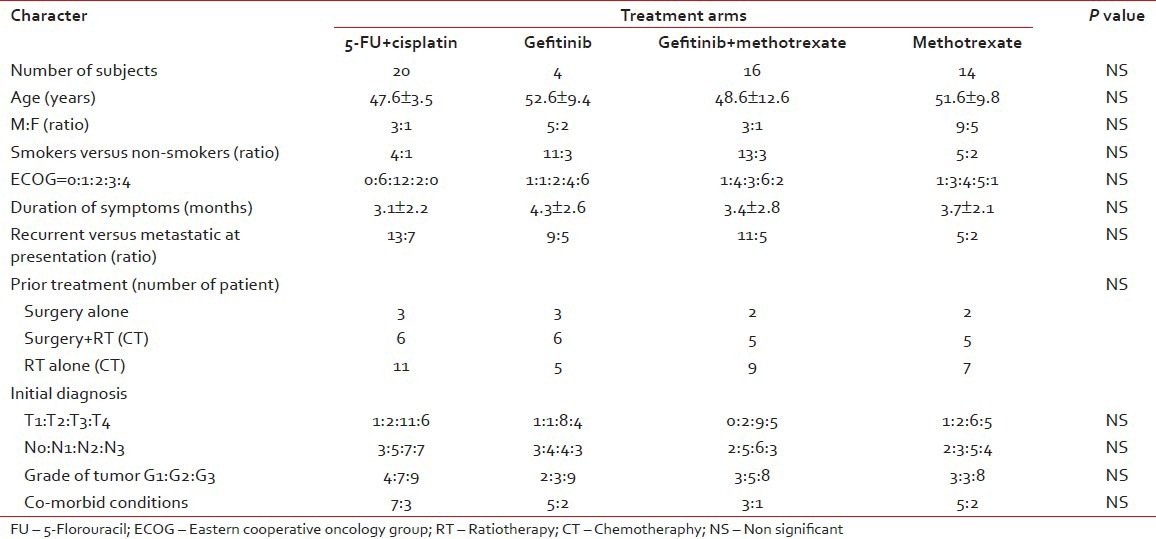
The baseline characters of the patients were represented in Table 1. Overall, the number of male patients is much higher than the female patients, which is in agreement with the published Indian literature. The major observation is the relatively higher number of patients with poor performance status in the gefitinib alone arm. There are no major differences in the median age, duration of symptoms, and distribution of different stage of disease between the four treatment groups. Though the numbers of patients in either group are not exactly the same, the sample size is too small to comment upon the differences.
Table 1
Baseline characters in the study
Toxicity profile and response rates
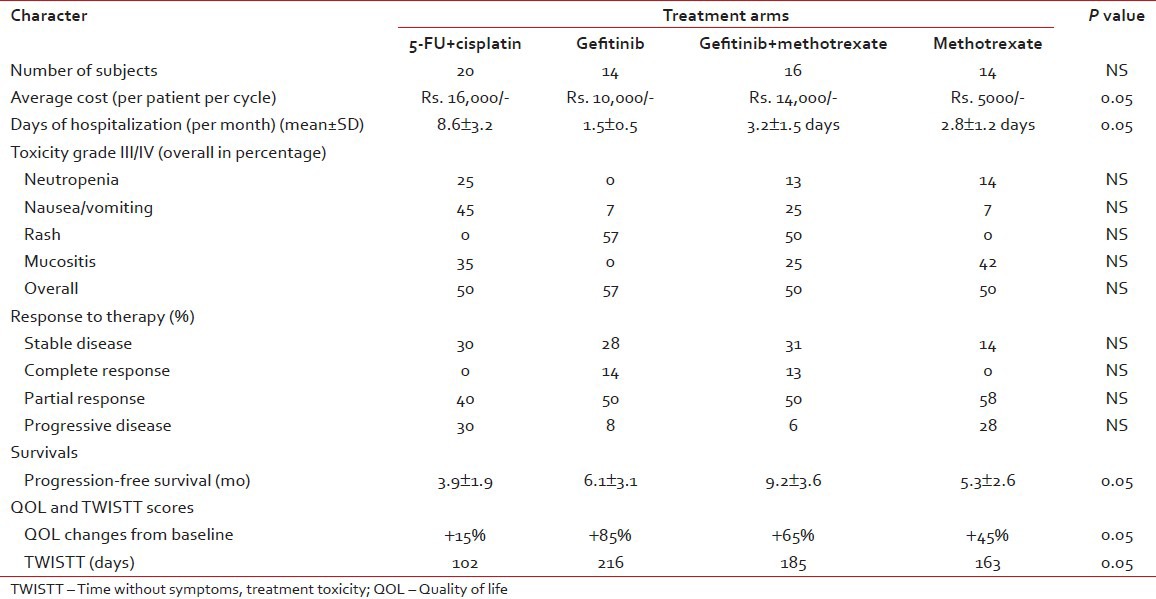
The overall grade III/IV toxicity observed in this study is 45%; however, when analyzed in each of the groups, patients receiving the 5-FU cisplatin had the maximum toxicity and those receiving the gefitinib had minimum toxicity. The exact details were represented in Table 2. Similarly, the differences observed with either regimen, as far as response rates are concerned, are not statistically significant, though methotrexate and gefitinib arm had higher overall response rates.
Table 2
Pharmcoeconomics, quality-of-life, response rates, and toxicity profile
Quality of life
The best observed effect of chemotherapy on the improvement of QOL is stabilization of symptoms and in some cases improving the same. However, among all the groups, the best effect was observed in methotrexate and gefitinib arm. This may be due to a combination of the better control of symptoms as well as lower toxicity rates observed with this regimen.
Pharmacoeconomics
There are no major differences in the overall cost of the either regimen. It was initially thought that the 5-FU cisplatin would have the minimum cost, but in contrary, it is the most expensive regimen in view of the prolonged hospital stay as well as higher complication rates. The cost in the gefitinib arm is not very high as we used the generic brand.
Follow-up and survival
The median follow-up of this study is 34 months and we studied the disease-free survival [Figure 1]. Gefitinib with methotrexate arm had shown a better disease-free survival compared to the other regimens. The survival subset analysis (responders to therapy vs. non-responders, well differentiated vs. poorly differentiated, patients with rash vs. without rash, etc.) was not done as these parameters are well known to show differences in previous studies and moreover the present sample size is not adequate to look for these differences and the aim of the study is mainly focused on QOL and PFS.
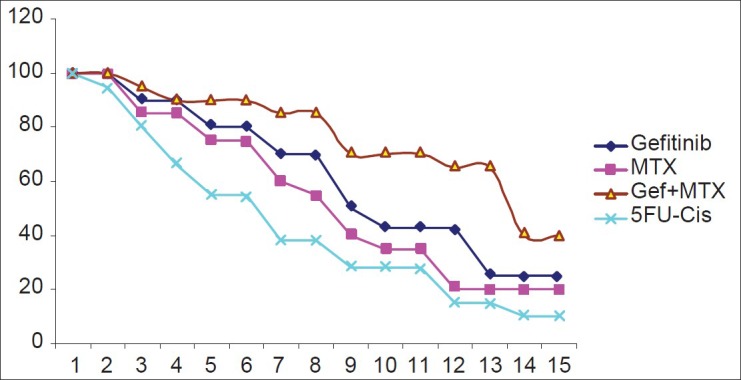
| Fig. 1 Kaplan-meier curves for survival period in four different arms
DISCUSSION
The limited treatment option, high morbidity, treatment-related toxicities, high incidence of recurrences, as well as costs involved in therapy makes head and neck squamous cell carcinoma an interested disease in our subcontinent. There is a recent shift in focus on disease stabilization with molecular-targeted drugs, in view of the earlier studies showing a benefit in survival as well as improved QOL in these patients. Of these molecules, gefitinib is promising as in phase II trials; in recurrent/metastatic HNSCC, it showed median Overall Survival (OS) and PFS of 6 and 3 months, respectively.[7,15] Even the response rates are impressive with 36% Overall Response Rate (ORR), in few other studies.[8] The recent review and meta-analysis showed that even drugs like methotrexate still have effect in disease control and are comparable to the other therapies with better QOL.[2] However, in this study, we observed a better disease control (ORR of around 50-60% compared to 30-40% in historical controls), probably due to the selection bias (where we expect a better follow-up in responders compared to non-responders, thereby reducing the denominator).
In view of the above observations, we selected four regimens, of which one (5-FU + cisplatin) is the gold standard. However, we did not study the taxane-based therapies or triplets as it could not be afforded by many (in fact upon reviewing all the hospital records during the one (1) year study period, we observed that we analyzed only 33% of the total patient pool, wherein remaining were either offered no chemotherapy [on best supportive care] or lost to follow-up) Our study demonstrates similar benefits with good palliation and improved PFS among the Methotrexate, gefitinib, and combination arms, which is superior to 5-FU + cisplatin arm and they are better tolerated. Our study supports the observation by Rao et al.[4] that gefitinib is effective even in patients with poor PS, in improving both QOL and survival, which questions the current inclusion criteria in many ongoing trails (where Eastern Cooperative Oncology Group (ECOG) Performance Scale of >1 are excluded by many trials). The most impressive finding is lack of any serious adverse reactions in the present population, which makes it an attractive option.
We also explored the combination of targeted therapy along with chemotherapy (based on many colon cancer and breast cancer trails, where Vascular endothelial growth factor (VEGF) and EGFR targeting agents were combined with chemotherapy and they consistently showed improved survival) and found that those using chemotherapy (MTX) along with gefitinib responded better (PFS of 9.2 months with 82% clinically benefit (SD + PR + CR) and better TWISTT scores, of 216 days) than any other regimens. As far as expected adverse events are concerned, rash was the commonest adverse effect observed, which did not require treatment in many. This is in contrast to the recent study by Stewart et al.[15] where the survival was not different in gefitinib 250 versus 500 versus combination with methotrexate arm and the survival in this study of 9 months is definitely more than those observed by them (6 months).
In conclusion, our data suggest that methotrexate weekly as single agent or in combination with gefitinib may be considered an alternative regimen in metastatic HNSCC patients with poor performance status, These observations if proved in a prospective manner, and got validated, may give a cost-effective alternative with better TWISTT score and QOL.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES

| Fig. 1 Kaplan-meier curves for survival period in four different arms


 PDF
PDF  Views
Views  Share
Share

