Paroxysmal Sympathetic Hyperactivity – An Under-Recognized Entity in Pediatric Brain Tumors: Case Report and Review of Literature
CC BY-NC-ND 4.0 · Indian J Med Paediatr Oncol 2020; 41(02): 254-256
DOI: DOI: 10.4103/ijmpo.ijmpo_93_18
Abstract
Paroxysmal sympathetic hyperactivity (PSH) is not a well-recognized syndrome in pediatric brain tumors, but has been described in adults with traumatic brain injury. We describe the case of a child with medulloblastoma presenting with PSH. An index of suspicion is important in early diagnosis of PSH and this ultimately has an impact on the long-term outcome of patients with the syndrome.
Keywords
Dysautonomia - Medulloblastoma - paroxysmal sympathetic hyperactivity - Pediatric brain tumorsPublication History
Received: 26 April 2018
Accepted: 21 June 2018
Article published online:
23 May 2021
© 2020. Indian Society of Medical and Paediatric Oncology. This is an open access article published by Thieme under the terms of the Creative Commons Attribution-NonDerivative-NonCommercial-License, permitting copying and reproduction so long as the original work is given appropriate credit. Contents may not be used for commercial purposes, or adapted, remixed, transformed or built upon. (https://creativecommons.org/licenses/by-nc-nd/4.0/.)
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India
Abstract
Paroxysmal sympathetic hyperactivity (PSH) is not a well-recognized syndrome in pediatric brain tumors, but has been described in adults with traumatic brain injury. We describe the case of a child with medulloblastoma presenting with PSH. An index of suspicion is important in early diagnosis of PSH and this ultimately has an impact on the long-term outcome of patients with the syndrome.
Keywords
Dysautonomia - Medulloblastoma - paroxysmal sympathetic hyperactivity - Pediatric brain tumorsIntroduction
Paroxysmal sympathetic hyperactivity (PSH) is a group of symptoms and signs suggestive of episodic autonomic hyperactivity and is not a very well-recognized entity in pediatric oncology as most reports are following acquired traumatic brain injury. There are also some published data of sympathetic hyperactivity in children with infections related to the brain. Although rare, an index of suspicion is important in the early diagnosis of this syndrome and this ultimately affects the long-term outcome. We report the case of a child with medulloblastoma with PSH.
Case Report
A 9 year old girl who presented with cerebellar signs was diagnosed with medulloblastoma (sonic hedgehog type)-standard risk after undergoing surgery for a fourth ventricle tumor. Although adjuvant chemoradiotherapy was advised, her parents did not comply with the treatment. Six months later, she had recurrence of symptoms and imaging confirmed relapse. She underwent a second debulking surgery but had persistent cerebellar signs. Four weeks after surgery, she was started on craniospinal radiotherapy.
After the first fraction of radiation, she developed persistent hiccups, tachycardia (>140), tachypnea, and hypertension (>95th centile). Supportive treatment with metoclopramide and baclofen improved symptoms for a short while and radiotherapy was continued.
After 1 week under radiation treatment, she presented with recurrent paroxysmal episodes of lethargy. Observations showed persistent tachycardia, tachypnea, hypertension, fever, and dystonia of the neck. Evaluation showed features suggestive of subacute intestinal obstruction with evidence of dilated bowel loops. Supportive treatments including correction of anemia with packed cell transfusion, possible sepsis with antibiotics, and bowel rest were initiated. Calcium channel blockers were started, but controlling hypertension was difficult. Investigations including renal function, liver function, electrolytes, and blood cultures were normal. Computed tomography pulmonary angiography did not show any evidence of pulmonary embolism. In view of difficulty in controlling symptoms, sympathetic hyperactivity was considered as a differential diagnosis and centrally acting sympathomimetic (clonidine) with a beta-blocker (propranolol) was initiated. There was a dramatic response in the control of signs and symptoms. The clinical picture and response to treatment favored a final diagnosis of PSH. She was continued on radiation and completed the treatment protocol without any major issues.
Discussion
PSH has been described in literature since 1954, known as autonomic seizures.[1] With more evidence emerging in the early 21st century, PSH became recognized as a definite entity though known by several names such as dysautonomia,[2] [3] hypothalamic midbrain dysregulation syndrome, autonomic storming, autonomic diencephalic epilepsy, and paroxysmal sympathetic instability with dystonia. Later, a consensus identified a name for the entity “paroxysmal sympathetic hyperactivity,” which was specific, was not preempting an underlying pathophysiology, and accurately portrayed the conceptual definition.[4]
PSH affects approximately 8%–10% of patients with acquired brain injuries, causing significant morbidities.[5] Apart from traumatic brain injury, other nontraumatic causes include brain tumors, infections, encephalitis, metabolic disorders, and hypoxic ischemic injury.[6] [7] Very little published data are available on childhood brain tumors. Goh et al. had described the phenomenon which developed within a week following resection of a midbrain glioma in a 7-year-old child and lasted for 6 months.[8] In children, there are some old reports of diencephalic autonomic epilepsy caused by a diencephalic neoplasm and arachnoid cyst.[9] Our child developed PSH following surgery and at the start of radiotherapy.
PSH has a host of possible signs and symptoms of sympathetic overactivity including increased body temperature, heart rate, respiratory function, blood pressure, sweating, agitation, muscle posturing (decerebrate and decorticate), and hypertonia (spasticity, rigidity, or dystonia). Previously, the presentation of five or more of the following symptoms was considered diagnostic of PSH: tachypnea, tachycardia, hyperthermia, hypertension, diaphoresis, dystonia, and decerebrate and decorticate posturing. In 2014, a PSH Consensus Working Group was formed for defining the various aspects of PSH. They put forward a clinical feature scale (CFS scale) [Table 1] and a diagnostic likelihood tool (DLT) [Table 2] for early diagnosis of PSH. A diagnostic likelihood score combining CFS and DLT more than 17 made the diagnosis of PSH more probable.[4] However, this score is not validated in children and a modified score needs to be formulated.
Table 1 Paroxysmal sympathetic hyperactivity assessment measure, clinical feature scale
Table 2Paroxysmal sympathetic hyperactivity assessment measure, diagnostic likelihood tool
|
Parameter |
Score |
||
|---|---|---|---|
|
CFS – Clinical feature scale; DLT – Diagnostic likelihood tool |
|||
|
Clinical features occur simultaneously |
1 |
||
|
Paroxysmal episodes |
1 |
||
|
Sympathetic overreactivity to painless stimuli |
1 |
||
|
Clinical features occur for >3 consecutive days |
1 |
||
|
Clinical features persist for >2 weeks following brain injury |
1 |
||
|
Clinical features persist after treatment of alternative differential diagnosis |
1 |
||
|
Number of attacks in a day >2 |
1 |
||
|
Absence of parasympathetic neural symptoms during attacks |
1 |
||
|
No other presumed cause of clinical features |
1 |
||
|
Antecedent-acquired brain injury |
1 |
||
|
PSH diagnostic likelihood |
Unlikely |
Likely |
Very likely |
|
Total score (CFS + DLT) |
less than 8 |
8-16 |
>17 |
In a child undergoing chemotherapy and radiotherapy, infectious processes such as pneumonia, sepsis, urinary tract infection and pulmonary embolism needs to be ruled out. Other differential diagnoses suggested are neuroleptic malignant syndrome, drug fever, seizure, rhabdomyolysis, and narcotic withdrawal. Development of symptoms may have a temporal relation to painful stimuli, suctioning, bathing, repositioning, etc. Constipation, distended bladder, abdominal distension, increased respiratory secretions, infections, pressure sore, or just an intravenous cannulation can trigger PSH. In our child, the only trigger we could identify was constipation following vincristine administration.
Pathophysiology of this condition is not clear. Initially thought to be epileptogenic in nature (autonomic epilepsy), no activity was seen in an electroencephalography. Current theories include conventional disconnection and excitatory: inhibitory ratio model which is an excessive adrenergic activity due to either disruption of pathways between the cortex and hypothalamus, which activates central excitatory foci around the diencephalon (thalamus and hypothalamus), or due to damage to the central inhibitory structures. In brain tumors, the additional etiology of autoimmunity also has been suggested.[10]
Immediate treatment of PSH is determining the urgency of control of symptoms. An algorithm has been suggested [Figure 1].[11] For children with airway, breathing, or circulation issues, administration of intravenous diazepam at a dose of 0.05–0.2 mg/kg/dose is recommended. Some authors have recommended clonazepam, hydroxyzine, and delorazepam. Use of intravenous morphine for poor response or intolerance to benzodiazepines has been suggested followed by addressing the precipitating conditions such as pain, constipation, or bladder distension. In a nonurgent situation, commonly advocated medications are beta-blockers (propranolol), central alpha blockers (clonidine), bromocriptine, gabapentin, benzodiazepine, medications for hyperthermia (baclofen and dantrolene), and opiates. Environmental modifications such as controlling the temperature of the room, limiting conversations inside the room, restricting visitors, and listening calm music have been suggested. Trying to have lights on during the day and reversing the same at night to establish regular sleep–awake cycle can limit the development of PSH.
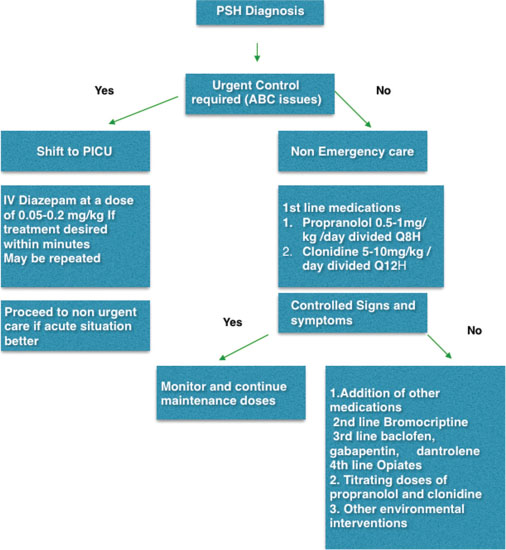
| Figure 1: Clinical management algorithm for paroxysmal sympathetic hyperactivity
Since PSH in children is associated with severe brain injury (low Glasgow Coma Scale), it has been associated with worse clinical outcome, prolonged periods of hospital stay, and intensive care support. Significance of the same in pediatric brain tumors needs to be studied. Uncontrolled sympathetic activity can lead to hemodynamic instability, neurogenic pulmonary edema, significant loss of weight, inadequate nutrition, and electrolyte imbalances. Abnormal posturing and dystonia increases the risk of heterotopic ossification. Early diagnosis and timely management has an impact on the long-term outcome.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Conflict of Interest
There are no conflicts of interest.
References
- Penfield W, Jasper H. Autonomic seizures. In: Penfield W, Jasper H. editors Epilepsy and the Functional Anatomy of the Human Brain. London: J & A Churchill Ltd; 1954: 412-37
- Kirk KA, Shoykhet M, Jeong JH, Tyler-Kabara EC, Henderson MJ, Bell MJ. et al. Dysautonomia after pediatric brain injury. Dev Med Child Neurol 2012; 54: 759-64
- Pranzatelli MR, Pavlakis SG, Gould RJ, De Vivo DC. Hypothalamic-midbrain dysregulation syndrome: Hypertension, hyperthermia, hyperventilation, and decerebration. J Child Neurol 1991; 6: 115-22
- Baguley IJ, Perkes IE, Fernandez-Ortega JF, Rabinstein AA, Dolce G, Hendricks HT. et al. Paroxysmal sympathetic hyperactivity after acquired brain injury: Consensus on conceptual definition, nomenclature, and diagnostic criteria. J Neurotrauma 2014; 31: 1515-20
- Letzkus L, Keim-Malpass J, Kennedy C. Paroxysmal sympathetic hyperactivity: Autonomic instability and muscle over-activity following severe brain injury. Brain Inj 2016; 30: 1181-5
- Farias-Moeller R, Carpenter JL, Dean N, Wells EM. Paroxysmal sympathetic hyperactivity in critically ill children with encephalitis and meningoencephalitis. Neurocrit Care 2015; 23: 380-5
- Xu Y, Wan L, Ning J, Guo W, Ren L. Paroxysmal sympathetic hyperactivity in a child with tuberculous meningitis A case study and review of related literature. West Indian Med J 2015; 64: 543-7
- Goh KY, Conway EJ, DaRosso RC, Muszynski CA, Epstein FJ. Sympathetic storms in a child with a midbrain glioma: A variant of diencephalic seizures. Pediatr Neurol 1999; 21: 742-4
- Giroud M, Sautreaux JL, Thierry A, Dumas R. Diencephalic epilepsy with congenital suprasellar arachnoid cyst in an infant. Childs Nerv Syst 1988; 4: 252-4
- Profile of autonomic dysfunctions in patients with primary brain tumor and possible autoimmunity. Clin Neurol Neurosurg 2016; 151: 51-4
- Burton JM, Morozova OM. Calming the storm: Dysautonomia for the pediatrician. Curr Probl Pediatr Adolesc Health Care 2017; 47: 145-50
Address for correspondence
Publication History
Received: 26 April 2018
Accepted: 21 June 2018
Article published online:
23 May 2021
© 2020. Indian Society of Medical and Paediatric Oncology. This is an open access article published by Thieme under the terms of the Creative Commons Attribution-NonDerivative-NonCommercial-License, permitting copying and reproduction so long as the original work is given appropriate credit. Contents may not be used for commercial purposes, or adapted, remixed, transformed or built upon. (https://creativecommons.org/licenses/by-nc-nd/4.0/.)
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India

| Figure 1: Clinical management algorithm for paroxysmal sympathetic hyperactivity
References
- Penfield W, Jasper H. Autonomic seizures. In: Penfield W, Jasper H. editors Epilepsy and the Functional Anatomy of the Human Brain. London: J & A Churchill Ltd; 1954: 412-37
- Kirk KA, Shoykhet M, Jeong JH, Tyler-Kabara EC, Henderson MJ, Bell MJ. et al. Dysautonomia after pediatric brain injury. Dev Med Child Neurol 2012; 54: 759-64
- Pranzatelli MR, Pavlakis SG, Gould RJ, De Vivo DC. Hypothalamic-midbrain dysregulation syndrome: Hypertension, hyperthermia, hyperventilation, and decerebration. J Child Neurol 1991; 6: 115-22
- Baguley IJ, Perkes IE, Fernandez-Ortega JF, Rabinstein AA, Dolce G, Hendricks HT. et al. Paroxysmal sympathetic hyperactivity after acquired brain injury: Consensus on conceptual definition, nomenclature, and diagnostic criteria. J Neurotrauma 2014; 31: 1515-20
- Letzkus L, Keim-Malpass J, Kennedy C. Paroxysmal sympathetic hyperactivity: Autonomic instability and muscle over-activity following severe brain injury. Brain Inj 2016; 30: 1181-5
- Farias-Moeller R, Carpenter JL, Dean N, Wells EM. Paroxysmal sympathetic hyperactivity in critically ill children with encephalitis and meningoencephalitis. Neurocrit Care 2015; 23: 380-5
- Xu Y, Wan L, Ning J, Guo W, Ren L. Paroxysmal sympathetic hyperactivity in a child with tuberculous meningitis A case study and review of related literature. West Indian Med J 2015; 64: 543-7
- Goh KY, Conway EJ, DaRosso RC, Muszynski CA, Epstein FJ. Sympathetic storms in a child with a midbrain glioma: A variant of diencephalic seizures. Pediatr Neurol 1999; 21: 742-4
- Giroud M, Sautreaux JL, Thierry A, Dumas R. Diencephalic epilepsy with congenital suprasellar arachnoid cyst in an infant. Childs Nerv Syst 1988; 4: 252-4
- Profile of autonomic dysfunctions in patients with primary brain tumor and possible autoimmunity. Clin Neurol Neurosurg 2016; 151: 51-4
- Burton JM, Morozova OM. Calming the storm: Dysautonomia for the pediatrician. Curr Probl Pediatr Adolesc Health Care 2017; 47: 145-50


 PDF
PDF  Views
Views  Share
Share

