Pattern of Adverse Drug Reactions to Anticancer Drugs: A Quantitative and Qualitative Analysis
CC BY-NC-ND 4.0 · Indian J Med Paediatr Oncol 2017; 38(02): 140-145
DOI: DOI: 10.4103/ijmpo.ijmpo_18_16
Abstract
Introduction: Anticancer drugs contribute significantly to the global burden of adverse drug reactions (ADRs). Any attempt to quantify their magnitude and provide upgraded knowledge would help oncologists in writing safer prescriptions. Aim: This observational follow-up study was conducted on newly diagnosed cancer patients receiving anticancer therapy with an aim to determine the frequency, severity, causality, predictability, and preventability of ADRs. Subjects and Methods: The patients were followed up for 6 months for the appearance of adverse events. Data were analyzed using IBM SPSS Statistics for Windows, Version 22.0. (Armonk, NY) and presented in the form of descriptive statistics. Results: Each patient was prescribed approximately 6.85 ± 1.51 (mean ± standard error) drugs on average. All the patients (100%) receiving anticancer chemotherapy had ADRs. Alopecia, nausea and vomiting, burning tingling, and numbness were the most frequently encountered ADRs. The incidence of alopecia (P < 0 class="i" xss=removed>P < 0 class="i" xss=removed>P < 0 class="b" xss=removed>Conclusion: Multiple ADRs were seen in newly diagnosed cancer patients. Most of them were predictable, of mild-to-moderate severity, nonserious, and preventable. A majority of the ADRs recovered over time.
Publication History
Article published online:
06 July 2021
© 2017. Indian Society of Medical and Paediatric Oncology. This is an open access article published by Thieme under the terms of the Creative Commons Attribution-NonDerivative-NonCommercial-License, permitting copying and reproduction so long as the original work is given appropriate credit. Contents may not be used for commercial purposes, or adapted, remixed, transformed or built upon. (https://creativecommons.org/licenses/by-nc-nd/4.0/.)
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India
Abstract
Introduction:
Anticancer drugs contribute significantly to the global burden of adverse drug reactions (ADRs). Any attempt to quantify their magnitude and provide upgraded knowledge would help oncologists in writing safer prescriptions.
Aim:
This observational follow-up study was conducted on newly diagnosed cancer patients receiving anticancer therapy with an aim to determine the frequency, severity, causality, predictability, and preventability of ADRs.
Subjects and Methods:
The patients were followed up for 6 months for the appearance of adverse events. Data were analyzed using IBM SPSS Statistics for Windows, Version 22.0. (Armonk, NY) and presented in the form of descriptive statistics.
Results:
Each patient was prescribed approximately 6.85 ± 1.51 (mean ± standard error) drugs on average. All the patients (100%) receiving anticancer chemotherapy had ADRs. Alopecia, nausea and vomiting, burning tingling, and numbness were the most frequently encountered ADRs. The incidence of alopecia (P < 0 xss=removed>P < 0 xss=removed>P < 0>
Conclusion:
Multiple ADRs were seen in newly diagnosed cancer patients. Most of them were predictable, of mild-to-moderate severity, nonserious, and preventable. A majority of the ADRs recovered over time.
Introduction
With an incidence of about 7%, adverse drug reactions (ADRs) are the fourth to sixth leading cause of death in hospitalized patients.[1] Drug toxicity also impacts the economy of health care negatively.[2] The drugs commonly associated with ADRs are antiepileptics, antineoplastics, antibiotics, anticoagulants, and nonsteroidal anti-inflammatory drugs. Among them, antineoplastic drugs are one of the most toxic drugs used in therapeutics.[3]
Anticancer therapy is hampered by the damage it inflicts on normal tissue. Moreover, cancer control with the available drugs has not been possible till date due to several reasons; mainly the intrinsic and extrinsic diversity among tumors. Cancer, meanwhile remains, a leading killer worldwide and anticancer drug development is a priority research area. New drugs come into the market after accelerated approval. With continued rise in the number of antineoplastics, the spectrum of ADRs associated with them has also diversified. During clinical trials, due to less number of study subjects, only commonly observed ADRs are reported. However, in postmarketing phase, more ADRs are observed. Moreover, the drug availability/use pattern, disease prevalence, ethnic diversity, and various environmental and geographical factors, etc.,[4] also determine the frequency, pattern, and severity of ADRs.
The selection of the ideal medicine for a given individual, by the prescribing practitioner, requires in-depth knowledge about its ADRs. Therefore, adequate information on ADRs of anticancer drugs and ways to ameliorate them should continuously be made available to oncologists. It is only then that the days of patients having to bear adverse effects and grin in the quest for tumor control will be over. Therefore, this study was planned to analyze the ADR profiles of anticancer drugs in patients attending the Oncology Department of a tertiary care institution.
Subjects and Methods
Patients being prescribed cancer chemotherapy for the first time, over a period of 12 months were included in the study. They were followed up every 21 days (when they came to receive chemotherapy) for at least 6 months, for occurrence of any adverse event. Patient's demographic details, and for each cycle, details of baseline investigations, anticancer treatment given, ADRs observed and interventions done to prevent and manage the ADRs were recorded. ADRs were analyzed in terms of frequency, severity, seriousness, grades, outcome, latency with start of chemotherapy, causality, preventability, and predictability in each cycle.
The drug groups/regimens and drug(s) prescribed most frequently, and those causing ADRs most frequently were determined. Grades were assigned to all the ADRs seen, on the basis of guidelines prepared by the National Cancer Institute-Common Toxicity Criteria (NCI-CTC). Probability assessment according to the Naranjo's algorithm was done by assigning the ADRs to a probability category from the total score obtained and also as per WHO - The Uppsala Monitoring Centre guidelines that classify ADRs as certain, probable, possible, unlikely and unclassifiable.[5,6] ADRs were classified as predictable or not predictable on the basis of modified guidelines developed by the Council for International Organizations of Medical Sciences. Preventability of the ADRs according to the modified Schumock–Thornton criteria was analyzed and categorized as definitely preventable, probably preventable, or not preventable.[7] ADRs were classified into various levels of severity as mild, moderate, and severe according to modified Hartwig severity scale.[8] Outcome of the ADRs as per WHO criteria as fatal, continuing, recovering, recovered, unknown, or any other was observed. The seriousness of ADRs categorized as per the WHO criteria as death, life-threatening, increased duration of hospitalization, disability, congenital anomaly, required intervention to prevent permanent impairment, was established. Reactions noticed for the first time in medical literature were also recorded.
Descriptive statistics were used to present the data. Interpretation and analysis of association of ADRs with various risk factors were carried out by application of Chi-square test to ascertain the level of significance wherever possible.
Results
A total of 200 newly diagnosed patients with 128 females and 72 males were included in the study. Mean weight of the 200 patients was 54.85 ± 12.62 kg. Mean age of all patients was 50.37 ± 13.77 years. Most of the patients fell in the age group of 51–60 years. Baseline characteristics were comparable as all patients were middle age, below average weight, prescribed 6–7 drugs and had about 4–5 ADRs on an average. Overall breast cancer (47%) followed by lung cancer (10%) was the commonest type of cancer [Figure 1].
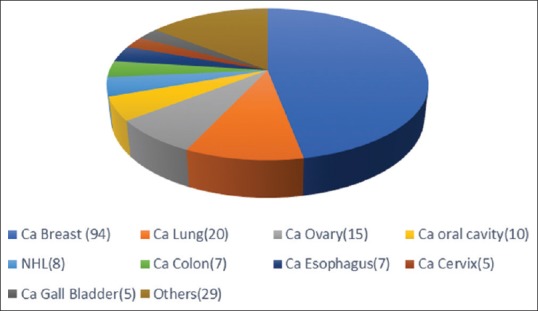
| Figure 1:Distribution of different types of cancers in the patient cohort (n=200). Other cancers: Ca Head of Pancreas (3), Unkown Primary- Ca (3), Ca Urinary Bladder (2), Ca Laryngopharynx (2), Ca Stomach (2), Ca Supraglottis (2), PDCA (2), Acute Lymphocytic Leukemia (1), Ca Hypopharynx (1), Ca Oropharynx (1), Ca Prostate (1), Ca Pyriform Fossa (1), Ca Rectum (1), Ca Testis: Germ Cell Tumour (1), Ewig's sarcoma (1), Hodgkin's Lymphoma (1), Malignant Fibrous Histiocytoma (1), Osteosarcoma Fibula (1), Pleomorphic Sarcoma back (1), Pleural Mesothelioma (1)
A mean ± S.E. of 6.85 ± 1.51 drugs was prescribed to all patients. Cyclophosphamide followed by 5-FU and Epirubicin were the most commonly used drugs [Table 1].
Table 1
Number of drugs prescribed and number of ADRs in males and females
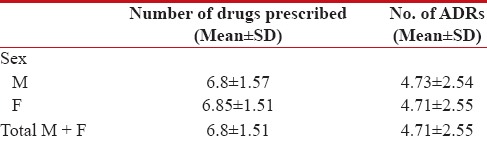
|
All patients suffered from ADRs. Overall a mean of 4.71 ± 2.55 ADRs were present; with no difference in the magnitude of ADRs seen between males and females [Table 1]. In males, maximum number of ADRs (5.23 ± 0.25) was seen in age group of 31–40 years. In females, maximum number of ADRs (4.95 ± 0.21) was seen in the age group of 71–80. All patients at the end of follow-up were alive except for the one male patient with lung cancer who died after his second cycle of chemotherapy.
Of the many reactions observed, Alopecia and nausea and vomiting were the commonest. Statistically significant difference was only present in the frequency of alopecia, nausea and oral ulceration between males and females [Figures [Figures22 and and3].3]. ADRs were seen in the gastrointestinal tract (40.55%) most frequently followed by skin (20.48%).
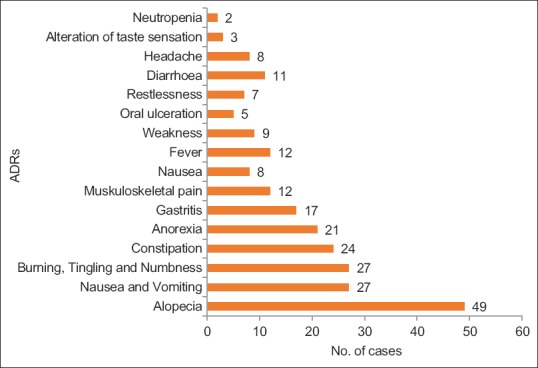
| Figure 2:Distribution of adverse events in Males (n=72). Alopecia, nausea, vomiting, PNS manifestations and constipation were the most common ADRs found in males
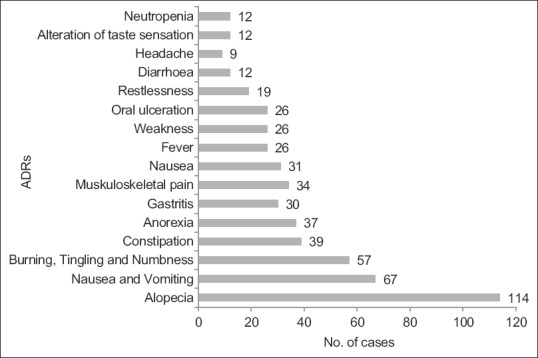
| Figure 3:Distribution of adverse events in Females (n=128). Alopecia, nausea, vomiting, PNS manifestations and constipation were the most common ADRs found in females
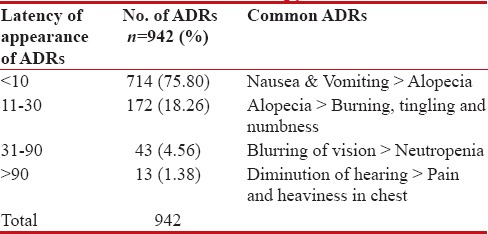
Nausea, vomiting, alopecia appeared the earliest; within ten days of start of chemotherapy, whereas diminution of hearing, blurring of vision etc. appeared later after a month [Table 2]. The appearance of anasarca on the 1st day of chemotherapy was the only unexpected finding in this study.
Table 2
Temporal Relation of ADRs with the start of chemotherapy
|
Most of the reactions were Grade 2 (NCI-CTC), not impairing the quality of life of individuals to a significant degree [Figure 4].
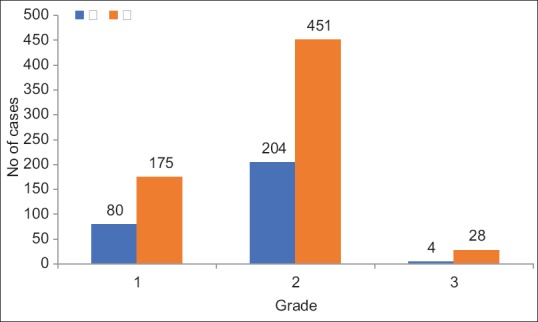
| Figure 4Grades of ADRs (n=942). Most of the reactions observed were of Grade 2
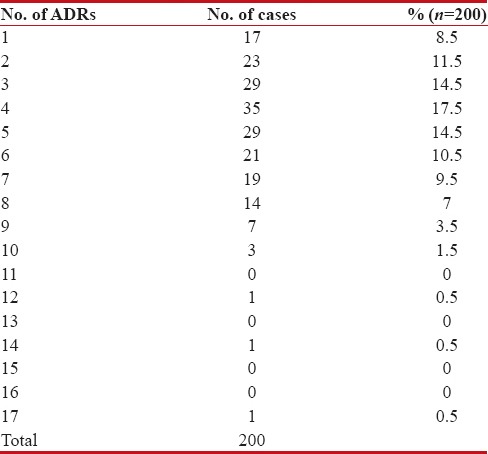
Maximum (17.5%) patients had a reported total four ADRs. 8.5% patients had only one ADR whereas one patient surprisingly had 17 ADRs. 1 to 8 ADRs were seen in most of the patients crossing double figures [Table 3].
Table 3
Number of ADRs observed in individual patients (n=200)
|
The reactions appearing commonly after every cycle (63%) were nausea and vomiting, gastritis, constipation, diarrhea, fever, peripheral nervous system (PNS) manifestations, alopecia, and musculoskeletal pain.
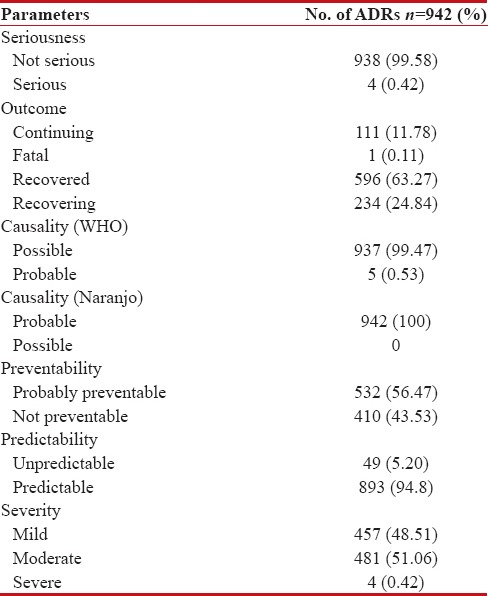
Most of the reactions (99.58%) were not serious. The serious reactions were mainly death (1), intractable nausea and vomiting (1), pain abdomen (1), and fever with chills and rigor (1), all requiring hospitalization/prolongation of stay in the hospital. Most reactions (63.27%) had recovered (e.g., nausea and vomiting, gastritis), 24.84% were recovering (e.g., alopecia, PNS manifestations), 11.78% had continuing reactions (e.g., weakness, anorexia, decrease in hearing), and one reaction was fatal (one death; which could have been drug or disease related) on the last follow-up.
When the predictability of ADRs was analyzed, 94.80% reactions were found to be predictable and 5.20% unpredictable. The important reactions that were found to be unpredictable were anasarca appearing on day 1 of chemotherapy, pharyngitis, allergic phenomena, breathlessness, sedation, hiccups, and death. Nearly 56.47% reactions were probably preventable, and 43.53% reactions were not preventable. According to modified Hartwig severity scale, 51.06% reactions were moderate, 48.51% mild, and 0.42% severe in intensity [Table 4].
Table 4
Seriousness, Outcome, Causality, Preventability, Predictability and Severity of ADRs
|
Discussion
Cancer cure today revolves around chemotherapy. Toxic anticancer drugs with low therapeutic index are routinely prescribed to more than 50%- cancer patients though their contribution to overall cure is only about 2%–5%. ADRs are considered an unavoidable component of cancer chemotherapy and are stoically accepted by both patients and health-care providers alike. The lack of a truly curative, safe drug, and the increasing burden of cancer necessitates rapid anticancer drug development and accelerated approval; manifesting as unknown toxicities. Many new targeted agents are being developed continuously.[9] The arbitrariness and variety in the chemotherapy regimens contribute further to ADRs, many of which can be prevented by the anticipatory prophylactic use of drugs.
All patients receiving chemotherapy in this study had an ADR; similar to the previous reports of 100%-patients receiving anticancer drugs having at least one ADR.[10,11] About four ADRs/patients were observed in this study, reflecting the normal range of ADRs appearing in cancer patients, i.e., 2–7.
Overall, a mean of 4.71 ± 2.55 ADRs was observed; surprisingly without any male or female preponderance, completely in contrast to the known and accepted concept that women are 50%–75%-more likely than men to experience an ADR. This probably occurs due to increased drug bioavailability, greater sensitivity to medications, lower body weight, lower organ sizes, higher-%-of body fat, lower volume of distribution as well as greater awareness in females than in males. Surely, the same applies to drug usage in oncology.[12,13]
The most common reaction observed was alopecia (Grade 2) seen in 81.5%-individuals; which is usually the case occurring in 30%–78%-individuals (Grades 2–3 severity).[14] Hair fall in most patients started after about 10 days, occurred gradually, and continued throughout chemotherapy. Cyclophosphamide followed by doxorubicin, cisplatin, and 5-FU were the most common drugs being used in these patients. Scalp cooling, scalp tourniquet, and 2%- minoxidil may prevent and attenuate chemotherapy-induced alopecia; none of these approaches were tried in these patients.[15,16]
Nausea and vomiting (Grade 2), occurring in 47%-individuals, was the second most common reaction. The previous studies have also reported the incidence of chemotherapy-induced nausea and vomiting (CINV) to be around 25%–100%.[17,18] CINV in the study population was mostly acute (85%). Cyclophosphamide followed by cisplatin and 5-FU were the common agents responsible in the present study for CINV. It occurred more frequently in women around the age of 45. History of light alcohol abuse, previous CINV, motion sickness, anxiety, and depression were the usual risk factors associated with CINV. All patients in this study received prophylactic ondansetron and dexamethasone for the prevention of CINV. Four patients also received aprepitant along with them. The combination of aprepitant, ondansetron, and dexamethasone controls CINV in 51%-patients compared to ondansetron and dexamethasone which prevents CINV in 42%-patients only.[19]
Alternating between appearing first and second, alopecia and nausea and vomiting have unanimously been reported as the commonest reactions to anticancer drugs.
The ADRs that have high documented incidence rates were also the ten most common ADRs in the present study.[7]
Whereas Grade 3 reactions are usually seen with anticancer drug therapy, most of the reactions in the present study were Grade 2 (69.53%).[2] Hence, a better quality of life can hesitantly be assumed for the patients in this study reflecting on a better physiological reserve of newly diagnosed cancer patients and also on the excellent management protocol in place.
It seems a sorry situation where out of about 95%-reactions that are predictable, we can prevent probably, only 56% -ADRs. Surprisingly, we have no ADRs that appear definitely preventable. This could be due to less attention being paid to the ADRs that could have been prevented by the appropriate use of prophylactic measures; establishing the huge scope for research and betterment in this area. This further stresses on the importance of having proper pharmacovigilance and dedicated preventive measures in place, to bridge this enormous gap between predictability and preventability. Alopecia (using cooling caps, tourniquets), nausea and vomiting (using newer drugs such as palonosetron, aprepitant and the older ones in appropriate dosages and durations), PNS manifestations (by vitamin supplementation, neurotrophic agent usage, etc.), and many other ADRs can been easily prevented by appropriate prophylactic measures.
Quite, a few general ADR studies have been conducted in India but very few studies specifically pertaining to the ADRs of anticancer drugs and that too with a sample size of 200 patients. In the present study, active surveillance, with possible hope of finding out the risk factors, incidence, and severity of ADRs of anticancer drugs, was done, which is far superior to passive surveillance that is commonly practiced. Patients were followed up for 6 months and not just for the duration of chemotherapy; so even ADRs appearing after the cessation of chemotherapy or continuing after that could be analyzed. Epidemiological/demographic parameters, cancer pattern, and drug regimens were also taken into account while analyzing the ADRs. All data pertaining to the preventive and treatment measures used for the ADRs were collected. Very few such studies have been conducted in India that has analyzed the outcome, severity, and temporal domain of ADRs of anticancer drugs.
Surprisingly, breast cancer in males was not that rare in Uttarakhand as it is worldwide. Females were suffering from tobacco-related problems such as lung cancer and oral cavity cancer. There was no female predilection in the appearance of ADRs; a fact not seen earlier in any anticancer studies. The appearance of anasarca on the 1st day of chemotherapy and the high incidence and continuing nature of PNS manifestations were also an unusual finding. The fact that some ADRs appear early and others late was also an important finding in this study that needs further inquiry. These unique observations reiterate the importance of more studies.
The most important finding was the huge gap between predictability and preventability of ADRs; clearly stressing the importance of better prevention strategies. An encouraging fact was the quantitative and qualitative (lesser ADRs of lower grades) improvement in the ADRs.
Possibly, the biggest limitation of this study was the fact that it suffered from recall bias. ADRs that appeared during the previous cycle or after the patients went home were reported by them at the time they came for the next cycle. Hence, in spite of active surveillance, the exact dates, nature, intensity, and frequency could not be fully ascertained. All the results and observations were based mostly on patient complaints and few pertinent laboratory investigations (blood, kidney function test, etc.) due to paucity of invasive blood monitoring for confirmation. Hence, a number of biochemical/investigational ADRs (liver function test, audiometric investigations, etc.) could not be ascertained. More risk factors such as menstrual history, seasonal variation, etc., could be analyzed in the future studies. Patients with end-organ damage and patients receiving concomitant radiation can be included in fresh studies to generate a complete picture.
Conclusions
There is a dearth of pharmacovigilance data pertaining to anticancer drugs despite their high potential for drug toxicity. So a focused monitoring of ADR profile of anticancer drugs is the need of the hour. This will also help in developing an Indian database pertaining to side effects of anticancer drugs helping drug regulatory agencies in policy decisions. Causality analysis for the anticancer ADRs needs attention, so that, drugs most frequently causing ADRs can be identified, and better alternatives developed By putting emphasis on detection, of ADRs associated with anticancer drugs, and their subsequent categorization on the basis of causality, predictability, preventability, severity and outcome, a veritable encyclopedia of information can be created locally which will guide clinicians in selecting the best possible combinations from the available anticancer drugs.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Lazarou J, Pomeranz BH, Corey PN. Incidence of adverse drug reactions in hospitalized patients: A meta-analysis of prospective studies. JAMA 1998;279:1200-5.
- Surendiran A, Balamurugan N, Gunaseelan K, Akhtar S, Reddy KS, Adithan C. Adverse drug reaction profile of cisplatin-based chemotherapy regimen in a tertiary care hospital in India: An evaluative study. Indian J Pharmacol 2010;42:40-3.
- Routledge PA, O'Mahony MS, Woodhouse KW. Adverse drug reactions in elderly patients. Br J Clin Pharmacol 2004;57:121-6.
- Nayak S, Acharjya B. Adverse cutaneous drug reaction. Indian J Dermatol 2008;53:2-8.
- The Use of WHO - UMC System for Standardised Case Causality Assessment. Available from: http://www.who-umc.org/graphics/24734.pdf. [Last accessed on 2015 May 20].
- Naranjo CA, Busto U, Sellers EM, Sandor P, Ruiz I, Roberts EA, et al. Amethod for estimating the probability of adverse drug reactions. Clin Pharmacol Ther 1981;30:239-45.
- Lau PM, Stewart K, Dooley M. The ten most common adverse drug reactions (ADRs) in oncology patients: Do they matter to you? Support Care Cancer 2004;12:626-33.
- Hartwig SC, Siegel J, Schneider PJ. Preventability and severity assessment in reporting adverse drug reactions. Am J Hosp Pharm 1992;49:2229-32.
- Narang AS, Desai DS. Anticancer drug development: Unique aspects of pharmaceutical development. Pharmaceutical Perspectives of Cancer Therapeutics. New York, USA: Bristol Myers Squibb Co; 2009 p. 49-89.
- Guo HJ, Ren F, Zhang D, Ji M. Monitoring report on 341 cases of adverse reactions caused by antitumor drugs. Afr J Microbiol Res 2012;6:3774-7.
- Vijayalakshmi MK, Palatty PL, Bhat P, Dinesh M. A comparative assessment of the ADR profile in various anti-cancer regimens. J Clin Diagn Res 2011;5:1209-13.
- Rademaker M. Do women have more adverse drug reactions? Am J Clin Dermatol 2001;2:349-51.
- Domecq C, Naranjo CA, Ruiz I, Busto U. Sex related variation in the frequency and characteristics of ADRs. Intern J Clin Pharmacol Ther Toxicol 1998;46:505-11.
- ;Trüeb RM. Chemotherapy-induced alopecia. Semin Cutan Med Surg 2009;28:11-4.
- Pesce A, Cassuto JP, Joyner MV, DuJardin P, Audoly P. Scalp tourniquet in the prevention of chemotherapy-induced alopecia. N Engl J Med 1978;298:1204-5.
- Tran D, Sinclair RD, Schwarer AP, Chow CW. Permanent alopecia following chemotherapy and bone marrow transplantation. Australas J Dermatol 2000;41:106-8.
- Hesketh PJ. Chemotherapy-induced nausea and vomiting. N Engl J Med 2008;358:2482-94.
- Gandara DR, Roila F, Warr D, Edelman MJ, Perez EA, Gralla RJ. Consensus proposal for 5HT3 antagonists in the prevention of acute emesis related to highly emetogenic chemotherapy. Dose, schedule, and route of administration. Support Care Cancer 1998;6:237-43.
- Hesketh PJ, Grunberg SM, Gralla RJ, Warr DG, Roila F, de Wit R, et al. The oral neurokinin-1 antagonist aprepitant for the prevention of chemotherapy-induced nausea and vomiting: A multinational, randomized, double-blind, placebo-controlled trial in patients receiving high-dose cisplatin – The Aprepitant Protocol 052 Study Group. J Clin Oncol 2003;21:4112-9.

| Figure 1:Distribution of different types of cancers in the patient cohort (n=200). Other cancers: Ca Head of Pancreas (3), Unkown Primary- Ca (3), Ca Urinary Bladder (2), Ca Laryngopharynx (2), Ca Stomach (2), Ca Supraglottis (2), PDCA (2), Acute Lymphocytic Leukemia (1), Ca Hypopharynx (1), Ca Oropharynx (1), Ca Prostate (1), Ca Pyriform Fossa (1), Ca Rectum (1), Ca Testis: Germ Cell Tumour (1), Ewig's sarcoma (1), Hodgkin's Lymphoma (1), Malignant Fibrous Histiocytoma (1), Osteosarcoma Fibula (1), Pleomorphic Sarcoma back (1), Pleural Mesothelioma (1)

| Figure 2:Distribution of adverse events in Males (n=72). Alopecia, nausea, vomiting, PNS manifestations and constipation were the most common ADRs found in males

| Figure 3:Distribution of adverse events in Females (n=128). Alopecia, nausea, vomiting, PNS manifestations and constipation were the most common ADRs found in females

| Figure 4Grades of ADRs (n=942). Most of the reactions observed were of Grade 2
References
- Lazarou J, Pomeranz BH, Corey PN. Incidence of adverse drug reactions in hospitalized patients: A meta-analysis of prospective studies. JAMA 1998;279:1200-5.
- Surendiran A, Balamurugan N, Gunaseelan K, Akhtar S, Reddy KS, Adithan C. Adverse drug reaction profile of cisplatin-based chemotherapy regimen in a tertiary care hospital in India: An evaluative study. Indian J Pharmacol 2010;42:40-3.
- Routledge PA, O'Mahony MS, Woodhouse KW. Adverse drug reactions in elderly patients. Br J Clin Pharmacol 2004;57:121-6.
- Nayak S, Acharjya B. Adverse cutaneous drug reaction. Indian J Dermatol 2008;53:2-8.
- The Use of WHO - UMC System for Standardised Case Causality Assessment. Available from: http://www.who-umc.org/graphics/24734.pdf. [Last accessed on 2015 May 20].
- Naranjo CA, Busto U, Sellers EM, Sandor P, Ruiz I, Roberts EA, et al. Amethod for estimating the probability of adverse drug reactions. Clin Pharmacol Ther 1981;30:239-45.
- Lau PM, Stewart K, Dooley M. The ten most common adverse drug reactions (ADRs) in oncology patients: Do they matter to you? Support Care Cancer 2004;12:626-33.
- Hartwig SC, Siegel J, Schneider PJ. Preventability and severity assessment in reporting adverse drug reactions. Am J Hosp Pharm 1992;49:2229-32.
- Narang AS, Desai DS. Anticancer drug development: Unique aspects of pharmaceutical development. Pharmaceutical Perspectives of Cancer Therapeutics. New York, USA: Bristol Myers Squibb Co; 2009 p. 49-89.
- Guo HJ, Ren F, Zhang D, Ji M. Monitoring report on 341 cases of adverse reactions caused by antitumor drugs. Afr J Microbiol Res 2012;6:3774-7.
- Vijayalakshmi MK, Palatty PL, Bhat P, Dinesh M. A comparative assessment of the ADR profile in various anti-cancer regimens. J Clin Diagn Res 2011;5:1209-13.
- Rademaker M. Do women have more adverse drug reactions? Am J Clin Dermatol 2001;2:349-51.
- Domecq C, Naranjo CA, Ruiz I, Busto U. Sex related variation in the frequency and characteristics of ADRs. Intern J Clin Pharmacol Ther Toxicol 1998;46:505-11.
- ;Trüeb RM. Chemotherapy-induced alopecia. Semin Cutan Med Surg 2009;28:11-4.
- Pesce A, Cassuto JP, Joyner MV, DuJardin P, Audoly P. Scalp tourniquet in the prevention of chemotherapy-induced alopecia. N Engl J Med 1978;298:1204-5.
- Tran D, Sinclair RD, Schwarer AP, Chow CW. Permanent alopecia following chemotherapy and bone marrow transplantation. Australas J Dermatol 2000;41:106-8.
- Hesketh PJ. Chemotherapy-induced nausea and vomiting. N Engl J Med 2008;358:2482-94.
- Gandara DR, Roila F, Warr D, Edelman MJ, Perez EA, Gralla RJ. Consensus proposal for 5HT3 antagonists in the prevention of acute emesis related to highly emetogenic chemotherapy. Dose, schedule, and route of administration. Support Care Cancer 1998;6:237-43.
- Hesketh PJ, Grunberg SM, Gralla RJ, Warr DG, Roila F, de Wit R, et al. The oral neurokinin-1 antagonist aprepitant for the prevention of chemotherapy-induced nausea and vomiting: A multinational, randomized, double-blind, placebo-controlled trial in patients receiving high-dose cisplatin – The Aprepitant Protocol 052 Study Group. J Clin Oncol 2003;21:4112-9.


 PDF
PDF  Views
Views  Share
Share

