Pediatric Bone Sarcomas: Outcome of Multimodality Treatment in a Single Institution in South India over a Decade
CC BY-NC-ND 4.0 ? Indian J Med Paediatr Oncol 2019; 40(S 01): S38-S43
DOI: DOI: 10.4103/ijmpo.ijmpo_235_17
Abstract
Context:?Pediatric bone sarcoma is a rare entity with low incidence of around 2.5?6 per million population in India. Management of this condition is well standardized, and global survival data are available; however, there is a paucity of data in the Indian perspective.?Aim of the Study:?The aim of this study is to analyze various prognostic factors and survival outcome. The purpose of this study is to assess the role of surgery, multiagent chemotherapy, and radiation in the management of these tumors. Patients and?Methods:?Retrospective analysis of patients aged 18 and less, diagnosed as bone sarcomas and treated in our tertiary cancer center. All the patients received at least one form of therapy depending on stage and site of the primary lesion.?Results:?Twenty-one patients of Ewing sarcoma and 20 patients of osteosarcomas were eligible and were included in the study. In Ewing sarcoma, completing the full course of standard chemotherapy and radiotherapy to the local site was associated with improved survival. In osteosarcoma, limb salvage surgery (LSS) had a significant difference in overall survival compared to amputation. Induction chemotherapy was associated with better percentage of necrosis and showed improved survival. The percentage of necrosis correlated positively with survival which was statistically significant (P?= 0.015).?Conclusion:?The median survival in both these bone sarcomas is inferior to global trends. Probable reasons for such discrepancy are lack of compliance to treatment protocols due to age factors and late presentation. Completion of multiagent chemotherapy in both the tumors add to better survival. Radiotherapy in Ewing sarcoma improves survival. In osteosarcoma, LSS is an oncologically safe alternative to amputation. The percentage of necrosis following chemotherapy in osteosarcoma is a reliable predictor of prognosis.
Publication History
Article published online:
24 May 2021
? 2019. Indian Society of Medical and Paediatric Oncology. This is an open access article published by Thieme under the terms of the Creative Commons Attribution-NonDerivative-NonCommercial-License, permitting copying and reproduction so long as the original work is given appropriate credit. Contents may not be used for commercial purposes, or adapted, remixed, transformed or built upon. (https://creativecommons.org/licenses/by-nc-nd/4.0/).
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India
Abstract
Context:?Pediatric bone sarcoma is a rare entity with low incidence of around 2.5?6 per million population in India. Management of this condition is well standardized, and global survival data are available; however, there is a paucity of data in the Indian perspective.?Aim of the Study:?The aim of this study is to analyze various prognostic factors and survival outcome. The purpose of this study is to assess the role of surgery, multiagent chemotherapy, and radiation in the management of these tumors. Patients and?Methods:?Retrospective analysis of patients aged 18 and less, diagnosed as bone sarcomas and treated in our tertiary cancer center. All the patients received at least one form of therapy depending on stage and site of the primary lesion.?Results:?Twenty-one patients of Ewing sarcoma and 20 patients of osteosarcomas were eligible and were included in the study. In Ewing sarcoma, completing the full course of standard chemotherapy and radiotherapy to the local site was associated with improved survival. In osteosarcoma, limb salvage surgery (LSS) had a significant difference in overall survival compared to amputation. Induction chemotherapy was associated with better percentage of necrosis and showed improved survival. The percentage of necrosis correlated positively with survival which was statistically significant (P?= 0.015).?Conclusion:?The median survival in both these bone sarcomas is inferior to global trends. Probable reasons for such discrepancy are lack of compliance to treatment protocols due to age factors and late presentation. Completion of multiagent chemotherapy in both the tumors add to better survival. Radiotherapy in Ewing sarcoma improves survival. In osteosarcoma, LSS is an oncologically safe alternative to amputation. The percentage of necrosis following chemotherapy in osteosarcoma is a reliable predictor of prognosis.
Introduction
Pediatric bone sarcoma is a rare entity with low incidence of around 2.5?6 per million population in India. Although there are lot of data on these tumours internationally, the data about the management and outcomes of these tumours in Indian perspective is abysmally low. Multimodality management of these tumors including aggressive multiagent systemic therapy, surgery in the form of limb salvage surgery (LSS) and radiation pose a great challenge to the treating multimodality team because patients usually present in an advanced stage in India. Compliance to treatment protocols directly reflect on the outcomes of the management of these tumors. The purpose of this study is to analyze the impact of various prognostic factors on survival outcomes.
Patients and Methods
This is a retrospective study of 10-year duration; data collection was done from previous medical records of patients treated in our institution and patients within the age group of 18 at presentation were included in this study. Totally, 21 patients of Ewing sarcoma and 20 patients of osteosarcoma were included in the study. The data collected were pooled and analyzed for management outcomes and prognostic association. Both these tumours are chemotherapy sensitive, patients were given standard accepted chemotherapy regimens and in some patients with slight alteration in the regimen. Standard regimen used in Ewing sarcoma was vincristine, adriamycin, and cyclophosphamide/ifosfamide etoposide (VAC/IE), some patients received Vincristine, Cyclophosphamide, Methotrexate/Cisplatin, Vincristine, Cyclophosphamide, Etoposide (VCM/PVCE). Osteosarcoma patients received AP with or without Ifosfamide, and some patients received PVCE. Although we insisted on strict adherence to the duration of treatment, especially chemotherapy, some patients defaulted treatment.
To analyze the data, SPSS (IBM SPSS Statistics for Windows, Version 22.0, IBM Corp. Released 2013, Armonk, NY, USA) was used. Correlation between survival time and percentage of necrosis was determined by Spearman Rank correlation. Significance level was fixed as 5% (? = 0.05). Various clinical data and treatment modalities were analyzed to know about their prognostic significance and their impact on survival. Finally, overall median survival and event-free survival were analyzed.
Results and Discussion
Ewing sarcoma and osteosarcoma account for around 5% of pediatric malignancies. The biological properties of these tumors are different; however, the treatment principles are similar.
Ewing sarcoma
Overall, the incidence of Ewing sarcoma is 2.93 per 1 million population. It is second most common bone tumor next to osteosarcoma. These tumors most commonly arise in the second decade of life. Most commonly, these tumors arise from the bone, but a small proportion, about 30% arises from the soft tissue. These tumors present clinically as localized pain of the affected bone and swelling; other nonspecific symptoms include fever loss of weight and appetite. The most common site of metastasis is lung followed by bone and bone marrow.
Classical radiographic findings are lytic or mixed lytic-sclerotic lesion, multilamellar periosteal reaction, namely onion peel appearance. Magnetic resonance imaging (MRI) and computed tomography (CT) scans are necessary to characterize and to establish the extent of the lesion locally. CT of the chest and bone scan (technetium 99 m) or a positron emission tomography (PET) scan for detecting bone metastasis. Bilateral bone marrow sampling is a must regardless of primary site or tumor size. Biopsy and histopathology are necessary to establish the diagnosis. Histologically, they are characterized by small round blue tumors, expressing CD99, and positive IHC for synaptophysin, Neuron-specific enolase, S100 and CD 57.
Overall, Ewing sarcoma has slight male preponderance. Of the 21 patients included for analysis, 12 were male, and nine were female, the difference in median survival of 2.5 months in favor of female gender was not statistically significant (P?= 0.499) [Table 1].
|
Factors |
Median/maximum survival months |
P |
|---|---|---|
|
Gender |
||
|
Male (n=12) |
14.5 |
0.499 |
|
Female (n=9) |
17.0 |
|
|
Location |
||
|
Axial (n=9) |
12 |
0.402 |
|
Appendicular (n=12) |
17 |
|
|
Stage |
||
|
IIB (n=14) |
16 |
0.417 |
|
III (n=1) |
3 |
|
|
IVB (n=6) |
10.5 |
|
|
Metastasis |
||
|
B/L lung (n=1) |
32 (maximum) |
Lung metastasis |
|
DL spine (n=1) |
6 (maximum) |
better survival |
|
Lung (n=2) |
32 (maximum) |
|
|
Skull (n=1) |
2 (maximum) |
|
|
Skull scapula pelvis (n=1) |
2 (maximum) |
|
|
Chemotherapy |
24 |
0.004 |
|
17 cycles (n=12) |
significant |
|
|
Incomplete (n=9) |
8 |
|
|
Radiation |
24.5 |
0.023 |
|
Received (n=12) |
significant |
|
|
Not received (n=9) |
13 |
|
|
Surgery |
||
|
Yes (n=6) |
16 |
0.704 |
|
No (n=15) |
15 |
|
|
Recurrence local (n=3) |
25 |
0.368 |
|
Distant (n=5) |
15 |
|
|
Histopathological response after surgery |
||
|
Partial (n=2) |
12.5 |
0.355 |
|
Total (n=4) |
22.5 |
|
|
Event-free survival |
11 19 |
|
|
Duration of survival |
15 |
|
|
39 (maximum) |
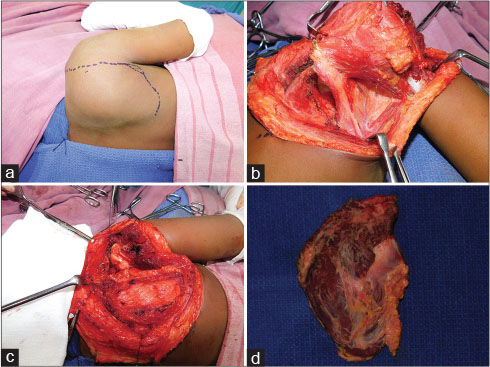
|?Figure. 51 (a)? Ewing Sarcoma of Scapula- after 6 cycles of chemotherapy. (b) Scapula with muscle attachments. (c) Humeral head suspended to the clavicle. (d) Scapula Specimen|
Among the patients treated with surgery, four patients had complete response, and two had partial response. Although there was a difference in median survival of 10 months (22.5 and 12.5 months respectively) between the two, it was not statistically significant (P?= 0.355). The survival difference was due to the response of the tumor to chemotherapy rather than surgery.
Totally 8 patients progressed to develop recurrence either local (n?= 3) and distant (n?= 5) with median survival of 25 and 15 months, respectively (P?= 0.368). Local recurrence had better median survival compared to distant recurrence though the difference was not statistically significant [Table 1].
Median event-free survival was 11 months and maximum of 19 months. Most of the recurrences were during this period of 1?1.5 years. Overall, median survival of Ewing sarcoma in our patients was 15 months with maximum survival of 39 months.
According Esiashvili?et al., the 5 years survival of localized disease increased from 44% to 68% in the period after 1993 whereas 5 years survival of metastatic disease increased from 16% to 39%.[4] However, survival in our group of patients is less compared to standards. The overall median survival is 15 months, and maximum survival is 39 months. Poor compliance on the part of patients, greater proportion of patients presenting with metastatic disease, and tumor biology in this age group are reasons for inferior survival outcomes. Completion of multiagent chemotherapy (VAC/IE) with the support of growth factors can improve survival.
Osteosarcoma
Osteosarcoma is the most common primary bone tumor. The peak incidence corresponds with the time of most rapid bone growth. The second peak of incidence after 60 years commonly referred to as secondary osteosarcoma. The disease is slightly more prevalent in males. From our observation, osteosarcoma is common in the second decade and common in males, 13 were male, and 7 were female, and there was no significant difference in survival between the two (P?= 0.812) [Table 2].
|
Factors |
Median/maximum survival months |
P |
|---|---|---|
|
*LSS ? Limb salvage Surgery; **IAP Ifosfamide, Adriamycin and Cisplatin |
||
|
Gender |
||
|
Male (n=13) |
17 |
0.812 |
|
Female (n=7) |
16 |
|
|
Site |
||
|
Femur (n=10) |
20.5 |
0.769 |
|
Tibia (n=7) |
17 |
|
|
Variant |
||
|
Osteoblastic (n=16) |
16.5 |
0.072 |
|
Chondroblastic (n=4) |
38 |
|
|
Response to chemotherapy (percentage of necrosis) |
||
|
Osteoblastic variant |
43% (mean) |
0.323 |
|
Chondroblastic variant |
61% (mean) |
|
|
Stage |
||
|
IIA (n=1) |
46 |
0.577 |
|
IIB (n=14) |
18 |
|
|
III (n=2) |
13.5 |
|
|
IVA (n=2) |
14 |
|
|
IVB (n=1) |
12 |
|
|
Metastasis bone (n=1) |
12 |
|
|
*LSS (n=2) |
14 |
|
|
Surgery LSS (n=12) |
20 |
0.044 |
|
Amputation (n=5) |
19 |
significant |
|
No surgery (n=3) |
3 |
|
|
Percentage of necrosis and correlation with survival |
Correlation 0.596 |
0.015 significant |
|
Chemotherapy complete (n=13) |
24 |
0.021 significant |
|
In complete (n=7) |
12 |
|
|
Chemotherapy regimen percentage of necrosis |
||
|
**IAP (n=12) |
70% |
0.048 |
|
Others (n=4) |
15% |
significant |
|
Event-free survival |
4 months (minimum) |
|
|
17 months (maximum) |
||
|
Duration of survival (months) |
17 (median) 58 (maximum) |
|
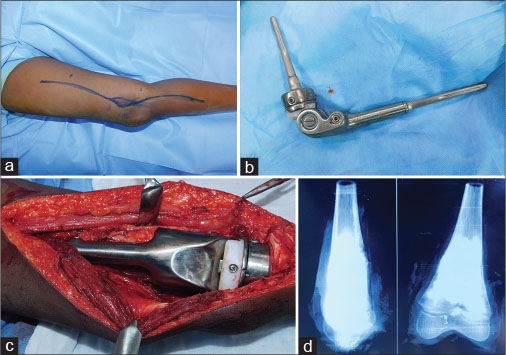
|?Figure. 2 (a)? Osteosarcoma of Distal Femur - after 3 cycles of neoadjuvant chemotherapy. (b) Expandable titanium distal femoral prosthesis. (c) Prosthesis secured by bone cement. (d) Specimen X-ray showing tumor response and margin|
Among patients undergoing LSS, eight patients had no complications 66.7%. The remaining four had complications. Dislocation after a total femoral prosthesis for which open reduction was done (n?= 1), Fracture of prosthesis and replacement (n?= 1), surgical site infection (n?= 1), vascular injury (n?= 1) for which an immediate reconstruction was done. One case of total femoral resection, during the postoperative period, developed dislocation of head of femur. As closed reduction failed, open reduction was done. Another patient had a fracture of the prosthesis, replacement of prosthesis was done. One patient had tumor abutting the proximal popliteal artery, LSS was done. Postoperatively, the patient had feeble distal pulse. Reexploration showed a thrombosed popliteal artery; a vein graft was done with contralateral saphenous vein. One more patient had surgical site infection which was then managed conservatively.
Thirteen patients completed full course of chemotherapy (Ifosfamide, Adriamycin and Cisplatin [IAP] based chemotherapy) and had a median survival of 24 months and those who defaulted chemotherapy had a median survival of 12 months the difference in survival was statistically significant (P?= 0.021) [Table 2]. Usually, we follow two drug regimens (doxorubicin, cisplatin with or without ifosfamide) with growth factors. The survival difference between those who received complete chemotherapy and those who did not was statistically significant, emphasizing the role of completeness of chemotherapy.
Average percentage of necrosis in patients receiving IAP-based chemotherapy was 70% and for those who received alternate regimen (PVCE) was 15% which was statistically significant (P?= 0.048). Spearman Rank correlation revealed that there was a strong positive correlation (0.59) between the percentage of necrosis and survival. Moreover, the correlation was statistically significant (P?= 0.015) [Table 2] and [3]. We were able to achieve median percentage of necrosis of 70% with (doxorubicin and cisplatin) compared to 15% in other regimens which was statistically significant. The reason for not achieving higher percentage of necrosis can be basically due to the biology of tumor or avoidance of high dose of methotrexate and at times Ifosfamide. The percentage of necrosis correlating with the survival was well established in our data too.
|
Chemotherapy |
Response 0-20 (%) |
21-40 |
41-60 |
61-80 |
81-100 |
|---|---|---|---|---|---|
|
Note: Four of 17 patients who received AP+/- Ifosfamide regimen were not operated so percentage of necrosis could not be measured.. AP ? Adriamycin, Cisplatin; PVCE ? Cisplatin, Vincristine, Cyclophosfamide, Etoposide |
|||||
|
*AP ? |
3 |
1 |
1 |
7 |
1 |
|
Ifosfamide (n=17) |
|||||
|
**PVCE (n=3) |
3 |
- |
- |
- |
- |
- Grier HE, Krailo MD, Tarbell NJ, Link MP, Fryer CJ, Pritchard DJ. et al.?Addition of ifosfamide and etoposide to standard chemotherapy for Ewing?s sarcoma and primitive neuroectodermal tumor of bone. N Engl J Med 2003; 348: 694-701
- Schuck A, Ahrens S, Paulussen M, Kuhlen M, K?nemann S, R?be C. et al.?Local therapy in localized Ewing tumors: Results of 1058 patients treated in the CESS 81, CESS 86, and EICESS 92 trials. Int J Radiat Oncol Biol Phys 2003; 55: 168-77
- Burgert Jr. EO, Nesbit ME, Garnsey LA, Gehan EA, Herrmann J, Vietti TJ. et al.?Multimodal therapy for the management of nonpelvic, localized Ewing?s sarcoma of bone: Intergroup study IESS-II. J Clin Oncol 1990; 8: 1514-24
- Esiashvili N, Goodman M, Marcus Jr. RB.?Changes in incidence and survival of Ewing sarcoma patients over the past 3 decades: Surveillance epidemiology and end results data. J Pediatr Hematol Oncol 2008; 30: 425-30
- Available from: http://www.cancer.net/cancer-types/osteosarcoma-childhood/statistics. [Last accessed on 2016 Nov].
- Canter RJ.?Chemotherapy: Does neoadjuvant or adjuvant therapy improve outcomes?. Surg Oncol Clin N Am 2016; 25: 861-72
Address for correspondence
Publication History
Article published online:
24 May 2021
? 2019. Indian Society of Medical and Paediatric Oncology. This is an open access article published by Thieme under the terms of the Creative Commons Attribution-NonDerivative-NonCommercial-License, permitting copying and reproduction so long as the original work is given appropriate credit. Contents may not be used for commercial purposes, or adapted, remixed, transformed or built upon. (https://creativecommons.org/licenses/by-nc-nd/4.0/).
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India

|?Figure. 51 (a)? Ewing Sarcoma of Scapula- after 6 cycles of chemotherapy. (b) Scapula with muscle attachments. (c) Humeral head suspended to the clavicle. (d) Scapula Specimen|
References


 PDF
PDF  Views
Views  Share
Share

