Peripheral and Central Giant Cell Lesions in Children: Institutional Experience at Subharti Dental College and Hospital
CC BY-NC-ND 4.0 · Indian J Med Paediatr Oncol 2017; 38(04): 440-446
DOI: DOI: 10.4103/ijmpo.ijmpo_17_16
Abstract
Introduction: Giant cell lesions (GCG) are a group of varied lesions that contain a multitude of multinucleated, osteoclast like giant cells within connective tissue stroma. These include giant cell granulomas which may be central (CGCG), if they lie within the jaw bone, or, peripheral (PGCG) if they lie within the soft tissue. Giant cell granulomascomprised 9.29% of all oral lesions. This case seriescomprises of 5 giant cell lesions in children between the ages of 4 to 12 years. Materials and Methods: A retrospective analysis was conducted of all patients who were diagnosed with giant cell lesions and treated over a period of 10 years (from August 2004 to August 2014) at Subharti Dental College and Hospital, Meerut, India. Results: A total of 5 giant cell lesions were identified in this case series, of which 2 cases were diagnosed as PGCG and 3 cases as CGCG. Surgical excision and curettage was performed for 2 peripheral lesions under local anesthesia while 1 central lesion was excised under general anesthesia. Two central lesions were treated with a non-surgical approach using intralesional corticosteroid. Conclusion: Our experience suggests that a correct diagnosis andcomplete surgical excision with curettage is effective incomplete management of oral giant cell lesions in the pediatric age group.
Publication History
Article published online:
04 July 2021
© 2017. Indian Society of Medical and Paediatric Oncology. This is an open access article published by Thieme under the terms of the Creative Commons Attribution-NonDerivative-NonCommercial-License, permitting copying and reproduction so long as the original work is given appropriate credit. Contents may not be used forcommercial purposes, or adapted, remixed, transformed or built upon. (https://creativecommons.org/licenses/by-nc-nd/4.0/.)
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India
Abstract
Introduction:
Giant cell lesions (GCG) are a group of varied lesions that contain a multitude of multinucleated, osteoclast like giant cells within connective tissue stroma. These include giant cell granulomas which may be central (CGCG), if they lie within the jaw bone, or, peripheral (PGCG) if they lie within the soft tissue. Giant cell granulomas comprised 9.29% of all oral lesions. This case series comprises of 5 giant cell lesions in children between the ages of 4 to 12 years.
Materials and Methods:
A retrospective analysis was conducted of all patients who were diagnosed with giant cell lesions and treated over a period of 10 years (from August 2004 to August 2014) at Subharti Dental College and Hospital, Meerut, India.
Results:
A total of 5 giant cell lesions were identified in this case series, of which 2 cases were diagnosed as PGCG and 3 cases as CGCG. Surgical excision and curettage was performed for 2 peripheral lesions under local anesthesia while 1 central lesion was excised under general anesthesia. Two central lesions were treated with a non-surgical approach using intralesional corticosteroid.
Conclusion:
Our experience suggests that a correct diagnosis and complete surgical excision with curettage is effective in complete management of oral giant cell lesions in the pediatric age group.
Introduction
Giant cell lesions (GCG) are a group of varied lesions that contain a multitude of multinucleated, osteoclast-like giant cells within connective tissue stroma. These include giant cell granulomas which may be central, if they lie within the jaw bone, or peripheral if they lie within the soft tissue. Giant cell granulomas comprised 9.29% of all oral lesions.[1]
Peripheral giant cell granuloma (PGCG) is a benign, reactive lesion that clinically presents as a soft to firm, reddish purple, polypoid, or nodular lesion. These lesions occur most frequently on the alveolar ridge or gingiva of the incisor or canine area of the mandible and originate from the connective tissue of the periosteum or the periodontal membrane.[2] The exact etiology of PGCG is uncertain, but it may also be associated with local irritating factors such as plaque, calculus, improper dental restorations, trauma, or tooth extractions.[3] These lesions are generally asymptomatic with unremarkable radiographic characteristics. However, adjacent tooth resorption may occasionally be seen radiographically. Although the lesion may occur at any age, PGCG lesions are more common in females in the fifth and sixth decade of life.[4] PGCG may also be associated with primary hyperparathyroidism or glycogen storage disease type Ib.[3]
Central giant cell granuloma (CGCG) is a benign, intraosseous lesion comprising 7% of all benign jaw lesions.[5] CGCG is defined as an intraosseous lesion consisting of cellular fibrous tissue that contains multiple foci of hemorrhage, aggregation of multinucleated giant cells, and occasionally trabeculae of woven bone.[6] CGCG shows a female predilection (2:1), is more common in the mandible than maxilla, with the anterior mandible the most common location and occurs predominantly before the age of 30 years.[6]
The clinical presentation of CGCG varies from a red or blue, soft, painless, asymptomatic swelling to a painful, aggressive lesion producing local osseous destruction, root resorption, tooth displacement, and/or cortical plate perforation with tendency for recurrence. Radiologically, the lesion shows wide variation and may present as a small, unilocular swelling or a large, multilocular lesion with root resorption, tooth displacement, and cortical perforation. Multiple CGCG lesions are uncommon and are usually associated with a syndrome such as cherubism, neurofibromatosis type 1 (NF1), or Noonan's syndrome.[7]
Histopathologic features of both the central and PGCG are characterized by the presence of abundant multinucleated giant cells and mononuclear stromal cells in a fibrous connective tissue. Hybrid lesions have also been reported which are CGCG lesions associated with fibro-osseous lesions mimicking GCG.[8] The aim of this case series is to describe the clinical features, histopathology, and management of 5 cases of giant cell granulomas in children.
Materials and Methods
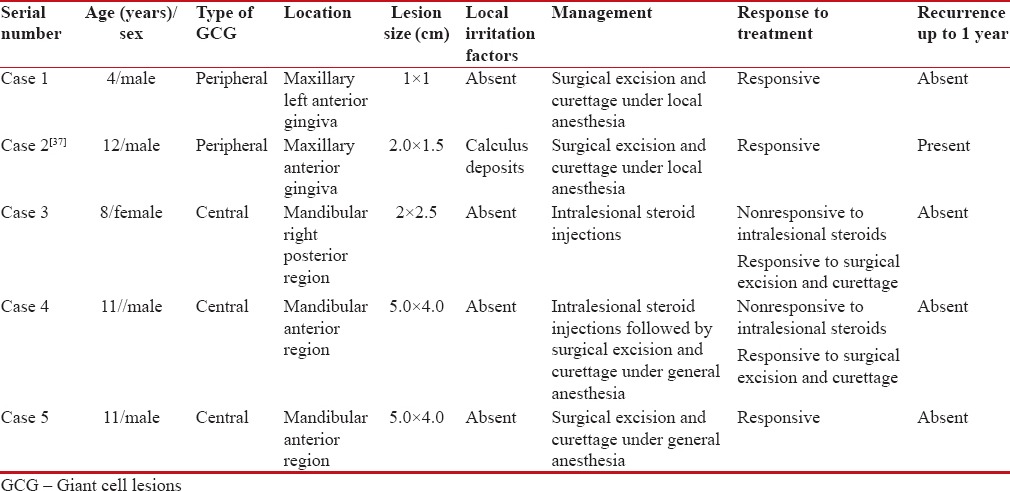
This case series comprises 5 GCG in children between the ages of 4 and 12 years. A retrospective analysis was conducted of all patients who were diagnosed with GCG and treated over 10 years (from August 2004 to August 2014) at Subharti Dental College and Hospital, Meerut, India. The lesions in these children were diagnosed and managed through interdisciplinary collaboration of the Departments of Oral and Maxillofacial Surgery, Oral Pathology and Microbiology and Pediatric Dentistry. Informed consent was obtained from the parents of all the children involved in the study. The analysis was approved by the Institutional Ethical Committee. The demographic details, clinical features, and management approaches of all the patients are listed in Table 1. Detailed history and clinical examination were performed for all the children. An orthopantomogram was taken for all the cases [Figures [Figures11 and and22].
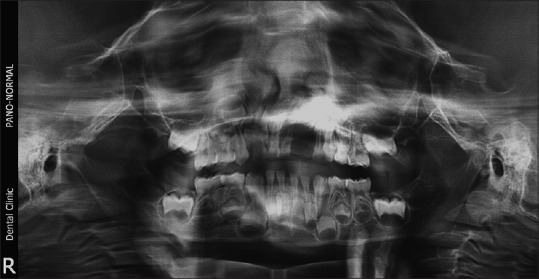
| Figure 1:Preoperative orthopantomogram showing peripheral giant cell lesion in the left maxilla

| Figure 2:Preoperative orthopantomogram showing radiolucent central giant cell granuloma of the mandible crossing the midline
Table 1
Demographic details, clinical features, and management approaches of all the patients
| Surgical excision and curettage were performed for two peripheral lesions under local anesthesia, whereas one central lesion was excised under general anesthesia. Adjacent teeth were scaled to remove local irritants. Two CGCG lesions were treated with a nonsurgical approach using intralesional corticosteroid. Medical conditions precluding the use of steroids were ruled out before therapy. A standard protocol[9] recommended in the literature was followed. Weekly injections of a mixture of equal parts of triamcinolone acetonide (Kenalog 10; 10 mg/ml) and local anesthetic (Xylocaine; 2% lignocaine hydrochloride with 1:200000 epinephrine) were used for 6 weeks. Response to treatment was assessed through: increasing difficulty of needle penetration into the lesion, back pressure felt while injecting solution, decreased swelling and pain, accompanied by increasing radiopacity visible on radiographs, and lesion regression.
Results
Out of the 5 GCG lesions identified [Table 1], 2 peripheral lesions were located in the maxillary anterior region. Three central lesions were, with 2 situated in the mandibular anterior region and 1 in the posterior region. Two of the central lesions crossed the midline. The size of the peripheral lesions was relatively small, varying from 1.0 cm × 1.0 cm to 2.0 cm × 1.5 cm while the central lesions varied in size from 2.0 cm × 2.5 cm to larger 5.0 cm × 4.0 cm lesions. The peripheral lesions were asymptomatic, soft, rubbery, and purplish red in color with one case showing calculus deposits on the adjacent teeth [Figure 3]. The central lesions were bluish red in color, hard with slight pain, or asymptomatic [Figure 4]. Radiographically, all the GCG identified lesions had a radiolucent cystic appearance, with variable levels of margin definition.
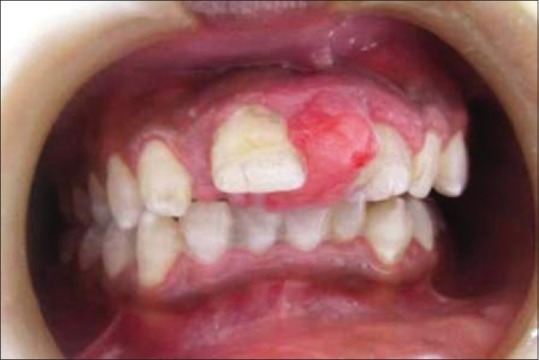
| Figure 3:Preoperative photograph showing peripheral giant cell granuloma lesion in relation to teeth number #11 and 21[37]
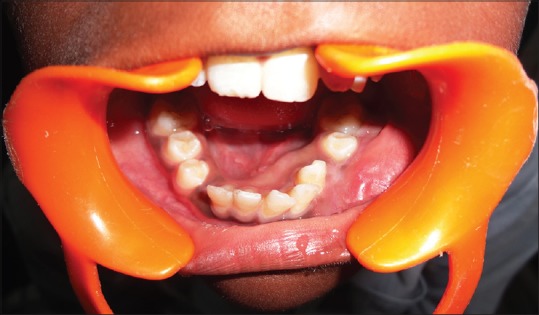
| Figure 4:Preoperative photograph showing central giant cell granuloma lesion in relation to teeth numbers #33–42
In all cases, a provisional diagnosis of giant cell granuloma was made on the basis of history and clinical examination. The serum calcium, phosphate, alkaline phosphatase, and parathyroid hormone (PTH) levels were undertaken to eliminate hyperparathyroidism in all cases. The histologic examination for patients 1 and 2 [Table 1] showed a nonencapsulated mass of tissue compiled of a fibrillar connective tissue stroma containing fibroblasts, multinucleated giant cells, blood vessels and hemosiderin pigmentation, suggestive of PGCG [Figure 5]. Histologically, for cases 3 through 5 [Table 1], cellular connective tissue stroma with numerous multinucleated giant cells, blood capillaries, bony trabeculae with osteocytes in lacunae, and mild lymphocytic infiltrate was seen on hematoxylin and eosin stained sections, suggestive of CGCG [Figure 6].
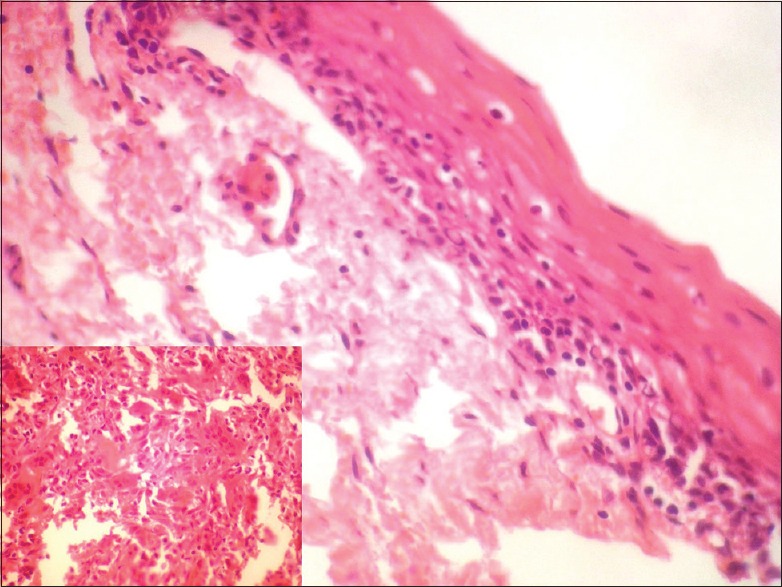
| Figure 5:Histopathologic picture of peripheral giant cell granuloma showing stratified squamous epithelium with an underlying fibrous, band-like stroma
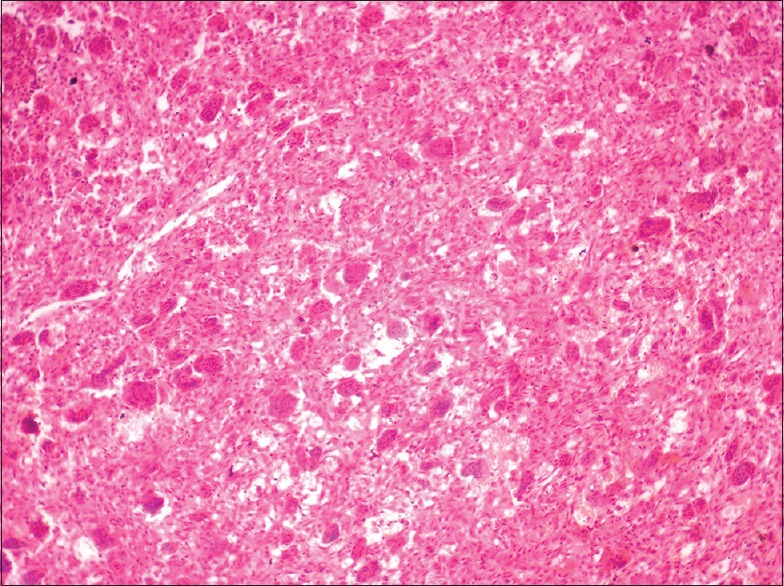
| Figure 6:Histopathologic view of central giant cell granuloma lesion showing fibrocellular connective tissue stroma with numerous multinuclear giant cells
Surgical excision and curettage were performed for two peripheral lesions and one central lesion. Adjacent teeth were scaled to remove local irritants. The postoperative healing was uneventful. One peripheral lesion showed recurrence at 8-month follow-up and was surgically re-excised. Two CGCG lesions were treated with a nonsurgical approach using intralesional corticosteroid. However, the lesions in these children were unresponsive to treatment after 6 weeks. Surgical excision and curettage of these lesions were later performed under general anesthesia. All the cases were reviewed on a monthly basis for 1-year postoperatively.
Discussion
A total of 5 GCG were identified in this case series, of which 2 cases were diagnosed as PGCG and 3 cases as CGCG. PGCG lesions occur more than 2 times more frequently than CGCGs.[1] Conversely, we encountered a slightly lesser number of PGCG cases (2) than CGCG (3) in this institutional experience.
PGCG is usually found in females and in adults, with few reported cases in children.[4] Only about 20%–33% of PGCG lesions are reported to occur in the first and second decades of life.[10] PGCG lesions are found to occur more often in females and in the mandibular anterior region. However, both of the PGCG lesions in our case series were male children, with maxillary anterior lesions. One study has shown equal prevalence of PGCG in both genders.[1] In children, reactive lesions such as PGCG can demonstrate a rapid growth rate and attain a large size over a relatively short period[11] and require prompt diagnosis and management. In addition, these soft-tissue nodules can cause superficial erosion of bone, produce minor or moderate tooth movement, and interfere with or delay eruption of teeth.[4]
Clinically, the PGCG lesions showed features consistent with the typical appearance of PGCG. Radiographically, no superficial erosion or tooth displacement was seen in our cases. The characteristic histologic features include a nonencapsulated highly cellular mass with abundant giant cells, inflammation, interstitial hemorrhage, hemosiderin deposits, mature bone or osteoid.[10] The histologic examination in our cases showed parakeratinized stratified squamous epithelium with fibrocellular connective tissue stroma showing numerous multinucleated giant cells, proliferating fibroblasts, blood vessels, and moderate amount of inflammatory cell infiltrate. Peripheral areas of the tissue showed few bony trabeculae and areas showing hemosiderin pigmentation were also evident.
The differential diagnosis of PGCG includes reactive gingival lesions such as peripheral ossifying fibroma (POF), pyogenic granuloma, and fibroma which present similar clinical and radiographic pictures. However, the pyogenic granuloma is friable and quick to bleed unlike PGCG, POFs, and fibromas lesions do not have the distinctive bluish-red color of PGCG lesions, and the parulis is characterized by purulent exudate and pain. Radiographically, POF may show evidence of dystrophic calcification, but all POF lesions do not routinely demonstrate this feature. These lesions may be differentiated further histologically. POFs are more cellular than fibromas and less vascular than pyogenic granulomas.[3] Hemangiomas may also mimic PGCG lesions but are distinguished from PGCG by brisk bleeding, increased warmth of the tissue, and blanching on palpation which are characteristic features of this vascular lesion.[11]
PGCG is managed by surgical excision and elimination of any local irritational factors. Timely diagnosis and intervention of PGCG lesions result in conservation of bone and tooth structure.[11] Regardless of the surgical technique employed for PGCG, it is important to eliminate etiologic factors, including plaque, calculus, and plaque-retentive restorations.[3] The recurrence rate is approximately 10%–17.5%, but multiple recurrences with eventual loss of the adjacent teeth are a potential complication.[11,12] Of importance to pediatric dentists treating PGCG, the recurrence rate mentioned refers to PGCG recurrence rates in children as well as adults. No clear data is available on recurrence rates in the pediatric population per se to the best of our knowledge. Recurrence was seen in one peripheral lesion in our series at 8-month follow-up and surgical retreatment was performed. The cause of recurrence in our case could be attributed to poor oral hygiene because recurring plaque and calculus deposits were present in the teeth adjacent to the lesion. Since PGCG is a reactive gingival lesion, these irritants may have triggered reformation of this lesion. A similar recurrence of PGCG lesion was reported by a few other studies.[1,13]
Although both peripheral and central GCGs are considered reactive lesions, the nature of CGCG is variable with various authors designating it as a reactive lesion, genetic abnormality with translocation of sex and autosomal chromosomes or as a benign tumor.[14] It was earlier termed as central giant cell reparative granuloma by Jaffe in 1953.[15] The reparative description of CGCG is currently considered obsolete, owing to the aggressive/destructive nature of the lesion.
CGCG occurs predominantly in females below the age of 30 years, favoring the mandibular anterior region.[6,16] In our series, all children presenting with CGCG were under the age of 30 years and 2 were female out of 3 cases. All CGCG lesions in our series were located in the mandibular anterior region, conforming to reported literature. CGCG lesions may also cross the midline, as was the case in 2 of our lesions.
The clinical behavior of CGCG is variable. Aggressive and nonaggressive lesions of CGCG may be distinguished by clinical, radiologic, or histologic features. Aggressive lesions are characterized by one or more of the following features: pain, paresthesia, root-resorption, rapid growth, cortical perforation, and a high recurrence rate following surgical curettage.[17,18] Aggressive lesions are also typically larger in size than nonaggressive ones. Our three cases of CGCG presented a nonaggressive picture without clinical symptoms. Radiographically, CGCG appears as a radiolucent defect, which may be unilocular or multilocular, and the defect is usually well-defined, as was in our cases. Aggressive lesions may show more root resorption and cortical perforation than nonaggressive ones.
The histologic picture of CGCG displays 2 distinctive features – a highly cellular, fibroblastic stroma with high vascular density and irregularly distributed giant cells, with variable cell size and morphology, within the stroma. Dystrophic calcification and metaplastic ossification are common, especially around the periphery of the lesion.[19] In our cases, similar histopathologic findings were observed, which showed cellular connective tissue stroma with numerous multinucleated giant cells, blood capillaries, bony trabeculae with osteocytes in lacunae, and mild lymphocytic infiltrate. Histologically, aggressive lesions display a larger fractional surface area occupied by giant cells.[17,18] The three CGCG lesions in this case series showed nonaggressive lesions with features conforming to the ones stated above. At a molecular level, the giant cells of CGCG are derived from a subset of mononuclear phagocytes. These mononuclear precursor cells differentiate into mature giant cells under the influence of RANKL-expressing, proliferating spindle-shaped (osteoblast-like) stroma cells (RANKL is an essential cytokine required for osteoclastogenesis).[20] However, immunohistochemistry is of limited use in differentiation between aggressive and nonaggressive lesions.
Ameloblastoma, odontogenic myxoma, giant cell tumors of long bones (GCT), Brown tumor of hyperparathyroidism, aneurysmal bone cyst, and GCG of genetic disorders such as cherubism, Noonan syndrome, and NF1 constitute the differential diagnosis of CGCG.[14] All of these lesions show multiple, lytic radiolucencies and are histologically and radiologically indistinguishable and a clinical-radiologic-biochemical approach is required to make a definitive diagnosis.[21] GCT occurs chiefly in long bone and less frequently affects the jaw bones, unlike CGCG. They are usually painful and fast growing. GCT and CGCG may represent a spectrum of a single disease process modified by the age of the patient and the site of occurrence.[22] Increased PTH levels can cause an imbalance between osteoclastic-osteoblastic homeostasis and calcium-phosphorus regulation which can lead to bone resorption with fibrous replacement of the marrow and thinning of the cortex. The Brown tumors are focal lesions found within these areas of bone resorption.[22] Patients showing GCG must undergo a serum parathyroid assay to rule out hyperparathyroidism. In some cases, the CGCG lesion may be the first indicator of the primary hyperparathyroidism.[23] Ameloblastoma tends to occur in an older age group (20–60 years), in the posterior mandible and shows well-defined internal septa radiographically. Conversely, CGCG shows wispy, ill-defined trabeculation. The odontogenic myxoma does not cause much expansion, occurs in an older age group and has well-defined septa. The aneurysmal bone cyst is typically composed of honeycomb-like, blood-filled spaces with a lining of flat nonendothelial cells, which distinguishes it from CGCG lesions. GCG in patients with Cherubism are generally accompanied by a family history of affected members and spontaneous lesion regression.[22] CGCG is only rarely present in patients with a Noonan genotype (mutation in PTPN11), a NF1 genotype or a neurofibromatosis-Noonan syndrome phenotype. This may mean that the occurrence of CGCG in these patients is coincidental.[22]
The accepted surgical management of CGCG lesions varies from simple curettage to wide en bloc resection depending on the following factors: clinical behavior, age of the patient, location and size of the lesion.[24] In young children, such as in our case, surgical treatments may lead to damage to primary teeth or to developing permanent tooth germs or teeth. One lesion out of the 3 CGCG lesions described here was treated with surgical excision and curettage only, whereas the remaining 2 lesions were surgically excised following intralesional steroid therapy.
Nonsurgical methods of treatment that have been tried involve using intralesional calcitonin,[22,25] intralesional steroids,[23,26,27] antiangiogenic therapy using interferons,[28] and radiotherapy.[29] The benefits of any nonsurgical treatment are that disfiguring surgery and the potential loss of teeth or tooth germs in children may be prevented.[26,27]
Steroids, such as triamcinolone acetonide, inhibit osteoclasts in marrow cultures and in conditions of resorption of bone by increased apoptosis. Successful nonsurgical treatment with intralesional corticosteroid injections has been advocated in a variety of cases.[24,26,27] The benefits of intralesional steroids include ease of administration, minimal invasiveness with no disfiguring or damaging outcomes, short duration of treatment, minimal side effects, and option to treat surgically at later date.[30] The disadvantages with intralesional steroid treatment are associated with the long treatment time, patient compliance, and systemic effects associated with the steroids used.[26] The vast majority of CGCG lesions subjected to initial intralesional corticosteroid treatment involve children, where surgery is potentially a more disfiguring and complicated option.[24] Intralesional steroids were used in two out of three cases of CGCG cases of CGCG discussed here. The steroidal therapy caused no untoward side effects and was well tolerated. However, both the lesions treated were nonresponsive to steroids after 6 weeks of intralesional steroid therapy using a standard protocol that recommends weekly injections of a mixture of equal parts of 10 mg/mL of triamcinolone acetonide and local anesthetic for 6 weeks. Surgical excision and curettage were later required for the definitive management. The possible reasons for nonresponse to steroid therapy in our cases could be the unpredictable response to intralesional steroid reported in some cases which required surgical intervention later.[30] This could have been minimized by receptor typing before selection of a particular nonsurgical treatment option. This involves identification of glucocorticoid or calcitonin receptors present on both mononuclear spindle cell and multinucleated giant cells.[30] CGCG lesions with unpredictable response to intralesional corticosteroids similar to our study have been reported in the literature.[9,30,31,32,33] Some authors recommend nonsurgical/corticosteroid therapy as first choice treatment in the management of CGCG lesions.[24,33]
The reported recurrence rate following surgical and nonsurgical treatment (isolated or combined use) varies among studies, ranging from 11% to 41%, with maxillary and aggressive lesions showing greater recurrence.[34,35] Whitaker and Waldron[35] reported a mean interval between diagnosis and initial treatment and treatment of a recurrence was 21 months and stated that very few recurrences manifested after 2 years of initial treatment. None of the CGCG lesions in our series showed recurrence up to 1 year of follow-up. Persistent recurrences can be explained by the reserved surgical procedures with regard to the germs of permanent teeth and the surrounding bone.[34] One author suggests resection of part of the jaw in all cases of central giant cell granuloma of the maxilla and sinuses, whereas in other localizations, they recommend curettage with grinding of the bone up to healthy tissue.[36] However, this type of resection would be a radical procedure involving a significant defect of part of the jaw and teeth[34] and such disfiguring treatments may be a less viable option in children.[37]
Conclusion
Our experience suggests that a correct diagnosis and complete surgical excision with curettage are effective in the complete management of oral GCG in the pediatric age group.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Motamedi MH, Eshghyar N, Jafari SM, Lassemi E, Navi F, Abbas FM, et al. Peripheral and central giant cell granulomas of the jaws: A demographic study. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2007;103:e39-43.
- Shadman N, Ebrahimi SF, Jafari S, Eslami M. Peripheral giant cell granuloma: A review of 123 cases. Dent Res J (Isfahan) 2009;6:47-50.
- Ozalp N, Sener E, Songur T. Peripheral giant cell granuloma and peripheral ossifying fibroma in children: Two case reports. Med Princ Pract 2010;19:159-62.
- Zhang W, Chen Y, An Z, Geng N, Bao D. Reactive gingival lesions: A retrospective study of 2,439 cases. Quintessence Int 2007;38:103-10.
- Kruse-Lösler B, Diallo R, Gaertner C, Mischke KL, Joos U, Kleinheinz J, et al. Central giant cell granuloma of the jaws: A clinical, radiologic, and histopathologic study of 26 cases. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2006;101:346-54.
- Neville BW, Damm DD, Allen CM, Bouquot JE. Oral and Maxillofacial Pathology. 4th ed. Philadelphia: Saunders; 2002. p. 705-40.
- Miloro M, Quinn PD. Synchronous central giant cell lesions of the jaws: Report of a case and review of the literature. J Oral Maxillofac Surg 1995;53:1350-5.
- Telfah H, Abdel Latif AM. Oral granulomas at prince Rashid Hospital North Jordan. A retrospective study of 62 cases. JRMS 2006;13:41-5.
- Terry BC, Jacoway JR. Management of central giant cell lesion: An alternative to surgical therapy. Oral Maxillofac Surg Clin North Am 1994;6:579-600.
- Katsikeris N, Kakarantza-Angelopoulou E, Angelopoulos AP. Peripheral giant cell granuloma. Clinicopathologic study of 224 new cases and review of 956 reported cases. Int J Oral Maxillofac Surg 1988;17:94-9.
- Flaitz CM. Peripheral giant cell granuloma: A potentially aggressive lesion in children. Pediatr Dent 2000;22:232-3.
- Lester SR, Cordell KG, Rosebush MS, Palaiologou AA, Maney P. Peripheral giant cell granulomas: A series of 279 cases. Oral Surg Oral Med Oral Pathol Oral Radiol 2014;118:475-82.
- Patil KB, Kalele KP, Kanakdande VD, Nayyar AS. Peripheral giant cell granuloma: Recurrence, a persisting problem. Int J Clin Case Rep 2015;5:1-6.
- Jadu FM, Pharoah MJ, Lee L, Baker GI, Allidina A. Central giant cell granuloma of the mandibular condyle: A case report and review of the literature. Dentomaxillofac Radiol 2011;40:60-4.
- Jaffe HL. Giant-cell reparative granuloma, traumatic bone cyst, and fibrous (fibro-oseous) dysplasia of the jawbones. Oral Surg Oral Med Oral Pathol 1953;6:159-75.
- Nicolai G, Lorè B, Mariani G, Bollero P, De Marinis L, Calabrese L, et al. Central giant cell granuloma of the jaws. J Craniofac Surg 2010;21:383-6.
- Ficarra G, Kaban LB, Hansen LS. Central giant cell lesions of the mandible and maxilla: A clinicopathologic and cytometric study. Oral Surg Oral Med Oral Pathol 1987;64:44-9.
- Chuong R, Kaban LB, Kozakewich H, Perez-Atayde A. Central giant cell lesions of the jaws: A clinicopathologic study. J Oral Maxillofac Surg 1986;44:708-13.
- Marx RE, Stern D. Oral and Maxillofacial Pathology. Chicago: Quintessence Publishing Co., Ltd.; 2002. p. 783-9.
- Miyamoto N, Higuchi Y, Tajima M, Ito M, Tsurudome M, Nishio M, et al. Spindle-shaped cells derived from giant-cell tumor of bone support differentiation of blood monocytes to osteoclast-like cells. J Orthop Res 2000;18:647-54.
- Lin YJ, Chen HS, Chen HR, Wang WC, Chen YK, Lin LM, et al. Central giant cell granuloma of the mandible in a 7-year-old boy: A case report. Quintessence Int 2007;38:253-9.
- de Lange J, Rosenberg AJ, van den Akker HP, Koole R, Wirds JJ, van den Berg H, et al. Treatment of central giant cell granuloma of the jaw with calcitonin. Int J Oral Maxillofac Surg 1999;28:372-6.
- Gulati D, Bansal V, Dubey P, Pandey S, Agrawal A. Central giant cell granuloma of posterior maxilla:First expression of primary hyperparathyroidism. Case Rep Endocrinol 2015;2015:170412.
- Sezer B, Koyuncu B, Gomel M, Günbay T. Intralesional corticosteroid injection for central giant cell granuloma: A case report and review of the literature. Turk J Pediatr 2005;47:75-81.
- Harris M. Central giant cell granulomas of the jaws regress with calcitonin therapy. Br J Oral Maxillofac Surg 1993;31:89-94.
- Khafif A, Krempl G, Medina JE. Treatment of giant cell granuloma of the maxilla with intralesional injection of steroids. Head Neck 2000;22:822-5.
- ;Kermer C, Millesi W, Watzke IM. Local injection of corticosteroids for central giant cell granuloma. A case report. Int J Oral Maxillofac Surg 1994;23:366-8.
- Kaban LB, Troulis MJ, Ebb D, August M, Hornicek FJ, Dodson TB, et al. Antiangiogenic therapy with interferon alpha for giant cell lesions of the jaws. J Oral Maxillofac Surg 2002;60:1103-11.
- Eisenbud L, Stern M, Rothberg M, Sachs SA. Central giant cell granuloma of the jaws: Experiences in the management of thirty-seven cases. J Oral Maxillofac Surg 1988;46:376-84.
- Mooney GC, McMahon J, Ward SE, Davidson LE, North S. Management of central giant cell granuloma: Discussion of two cases. Int J Paediatr Dent 2007;17:139-44.
- Adornato MC, Paticoff KA. Intralesional corticosteroid injection for treatment of central giant-cell granuloma. J Am Dent Assoc 2001;132:186-90.
- Carlos R, Sedano HO. Intralesional corticosteroids as an alternative treatment for central giant cell granuloma. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2002;93:161-6.
- Dolanmaz D, Esen A, Mihmanlı A, Işık K. Management of central giant cell granuloma of the jaws with intralesional steroid injection and review of the literature. Oral Maxillofac Surg 2016;20:203-9.
- Knežević G, Jokić D, Knežević D. Central giant cell granuloma of aggressive growth: Case presentation after monitoring for several years. Acta Stomatol Croat 2009;43:52-9.
- Whitaker SB, Waldron CA. Central giant cell lesions of the jaws. A clinical, radiologic, and histopathologic study. Oral Surg Oral Med Oral Pathol 1993;75:199-208.
- Stolovitzky JP, Waldron CA, McConnel FM. Giant cell lesions of the maxilla and paranasal sinuses. Head Neck 1994;16:143-8.
- Adlakha VK, Chandna P, Rehani U, Rana V, Malik P. Peripheral giant cell granuloma. J Indian Soc Pedod Prev Dent 2010;28:293-6.

| Figure 1:Preoperative orthopantomogram showing peripheral giant cell lesion in the left maxilla

| Figure 2:Preoperative orthopantomogram showing radiolucent central giant cell granuloma of the mandible crossing the midline

| Figure 3:Preoperative photograph showing peripheral giant cell granuloma lesion in relation to teeth number #11 and 21[37]

| Figure 4:Preoperative photograph showing central giant cell granuloma lesion in relation to teeth numbers #33–42

| Figure 5:Histopathologic picture of peripheral giant cell granuloma showing stratified squamous epithelium with an underlying fibrous, band-like stroma

| Figure 6:Histopathologic view of central giant cell granuloma lesion showing fibrocellular connective tissue stroma with numerous multinuclear giant cells
References
- Motamedi MH, Eshghyar N, Jafari SM, Lassemi E, Navi F, Abbas FM, et al. Peripheral and central giant cell granulomas of the jaws: A demographic study. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2007;103:e39-43.
- Shadman N, Ebrahimi SF, Jafari S, Eslami M. Peripheral giant cell granuloma: A review of 123 cases. Dent Res J (Isfahan) 2009;6:47-50.
- Ozalp N, Sener E, Songur T. Peripheral giant cell granuloma and peripheral ossifying fibroma in children: Two case reports. Med Princ Pract 2010;19:159-62.
- Zhang W, Chen Y, An Z, Geng N, Bao D. Reactive gingival lesions: A retrospective study of 2,439 cases. Quintessence Int 2007;38:103-10.
- Kruse-Lösler B, Diallo R, Gaertner C, Mischke KL, Joos U, Kleinheinz J, et al. Central giant cell granuloma of the jaws: A clinical, radiologic, and histopathologic study of 26 cases. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2006;101:346-54.
- Neville BW, Damm DD, Allen CM, Bouquot JE. Oral and Maxillofacial Pathology. 4th ed. Philadelphia: Saunders; 2002. p. 705-40.
- Miloro M, Quinn PD. Synchronous central giant cell lesions of the jaws: Report of a case and review of the literature. J Oral Maxillofac Surg 1995;53:1350-5.
- Telfah H, Abdel Latif AM. Oral granulomas at prince Rashid Hospital North Jordan. A retrospective study of 62 cases. JRMS 2006;13:41-5.
- Terry BC, Jacoway JR. Management of central giant cell lesion: An alternative to surgical therapy. Oral Maxillofac Surg Clin North Am 1994;6:579-600.
- Katsikeris N, Kakarantza-Angelopoulou E, Angelopoulos AP. Peripheral giant cell granuloma. Clinicopathologic study of 224 new cases and review of 956 reported cases. Int J Oral Maxillofac Surg 1988;17:94-9.
- Flaitz CM. Peripheral giant cell granuloma: A potentially aggressive lesion in children. Pediatr Dent 2000;22:232-3.
- Lester SR, Cordell KG, Rosebush MS, Palaiologou AA, Maney P. Peripheral giant cell granulomas: A series of 279 cases. Oral Surg Oral Med Oral Pathol Oral Radiol 2014;118:475-82.
- Patil KB, Kalele KP, Kanakdande VD, Nayyar AS. Peripheral giant cell granuloma: Recurrence, a persisting problem. Int J Clin Case Rep 2015;5:1-6.
- Jadu FM, Pharoah MJ, Lee L, Baker GI, Allidina A. Central giant cell granuloma of the mandibular condyle: A case report and review of the literature. Dentomaxillofac Radiol 2011;40:60-4.
- Jaffe HL. Giant-cell reparative granuloma, traumatic bone cyst, and fibrous (fibro-oseous) dysplasia of the jawbones. Oral Surg Oral Med Oral Pathol 1953;6:159-75.
- Nicolai G, Lorè B, Mariani G, Bollero P, De Marinis L, Calabrese L, et al. Central giant cell granuloma of the jaws. J Craniofac Surg 2010;21:383-6.
- Ficarra G, Kaban LB, Hansen LS. Central giant cell lesions of the mandible and maxilla: A clinicopathologic and cytometric study. Oral Surg Oral Med Oral Pathol 1987;64:44-9.
- Chuong R, Kaban LB, Kozakewich H, Perez-Atayde A. Central giant cell lesions of the jaws: A clinicopathologic study. J Oral Maxillofac Surg 1986;44:708-13.
- Marx RE, Stern D. Oral and Maxillofacial Pathology. Chicago: Quintessence Publishing Co., Ltd.; 2002. p. 783-9.
- Miyamoto N, Higuchi Y, Tajima M, Ito M, Tsurudome M, Nishio M, et al. Spindle-shaped cells derived from giant-cell tumor of bone support differentiation of blood monocytes to osteoclast-like cells. J Orthop Res 2000;18:647-54.
- Lin YJ, Chen HS, Chen HR, Wang WC, Chen YK, Lin LM, et al. Central giant cell granuloma of the mandible in a 7-year-old boy: A case report. Quintessence Int 2007;38:253-9.
- de Lange J, Rosenberg AJ, van den Akker HP, Koole R, Wirds JJ, van den Berg H, et al. Treatment of central giant cell granuloma of the jaw with calcitonin. Int J Oral Maxillofac Surg 1999;28:372-6.
- Gulati D, Bansal V, Dubey P, Pandey S, Agrawal A. Central giant cell granuloma of posterior maxilla:First expression of primary hyperparathyroidism. Case Rep Endocrinol 2015;2015:170412.
- Sezer B, Koyuncu B, Gomel M, Günbay T. Intralesional corticosteroid injection for central giant cell granuloma: A case report and review of the literature. Turk J Pediatr 2005;47:75-81.
- Harris M. Central giant cell granulomas of the jaws regress with calcitonin therapy. Br J Oral Maxillofac Surg 1993;31:89-94.
- Khafif A, Krempl G, Medina JE. Treatment of giant cell granuloma of the maxilla with intralesional injection of steroids. Head Neck 2000;22:822-5.
- ;Kermer C, Millesi W, Watzke IM. Local injection of corticosteroids for central giant cell granuloma. A case report. Int J Oral Maxillofac Surg 1994;23:366-8.
- Kaban LB, Troulis MJ, Ebb D, August M, Hornicek FJ, Dodson TB, et al. Antiangiogenic therapy with interferon alpha for giant cell lesions of the jaws. J Oral Maxillofac Surg 2002;60:1103-11.
- Eisenbud L, Stern M, Rothberg M, Sachs SA. Central giant cell granuloma of the jaws: Experiences in the management of thirty-seven cases. J Oral Maxillofac Surg 1988;46:376-84.
- Mooney GC, McMahon J, Ward SE, Davidson LE, North S. Management of central giant cell granuloma: Discussion of two cases. Int J Paediatr Dent 2007;17:139-44.
- Adornato MC, Paticoff KA. Intralesional corticosteroid injection for treatment of central giant-cell granuloma. J Am Dent Assoc 2001;132:186-90.
- Carlos R, Sedano HO. Intralesional corticosteroids as an alternative treatment for central giant cell granuloma. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2002;93:161-6.
- Dolanmaz D, Esen A, Mihmanlı A, Işık K. Management of central giant cell granuloma of the jaws with intralesional steroid injection and review of the literature. Oral Maxillofac Surg 2016;20:203-9.
- Knežević G, Jokić D, Knežević D. Central giant cell granuloma of aggressive growth: Case presentation after monitoring for several years. Acta Stomatol Croat 2009;43:52-9.
- Whitaker SB, Waldron CA. Central giant cell lesions of the jaws. A clinical, radiologic, and histopathologic study. Oral Surg Oral Med Oral Pathol 1993;75:199-208.
- Stolovitzky JP, Waldron CA, McConnel FM. Giant cell lesions of the maxilla and paranasal sinuses. Head Neck 1994;16:143-8.
- Adlakha VK, Chandna P, Rehani U, Rana V, Malik P. Peripheral giant cell granuloma. J Indian Soc Pedod Prev Dent 2010;28:293-6.


 PDF
PDF  Views
Views  Share
Share

