Pilomatrix Carcinoma Masquerading as Breast Carcinoma
CC BY-NC-ND 4.0 · Indian J Med Paediatr Oncol 2017; 38(03): 367-370
DOI: DOI: 10.4103/ijmpo.ijmpo_118_16
Abstract
Pilomatrix carcinoma is an exceedingly rare skin adnexal neoplasm derived from piliferous follicles, usually occurring in the head and neck region. Localization of this tumor in the breast is a rarity. We now report an unusual case of a 49-year-old female who presented with a palpable mass in the left breast for 2 years. Mammogram revealed a large, lobulated opacity with calcification, and positron emission tomography–computed tomography showed a metabolically active soft tissue mass measuring 15 cm involving all the quadrants of the left breast. Subsequently, the patient underwent radical mastectomy, and histopathologic diagnosis of pilomatrix carcinoma of the breast was offered. Pilomatrix carcinoma, although exceedingly rare, can have an intramammary location and can be misdiagnosed as breast carcinoma on limited material. A high index of suspicion is required to arrive at an accurate diagnosis so as to obviate neoadjuvant chemotherapy.
Publication History
Article published online:
04 July 2021
© 2017. Indian Society of Medical and Paediatric Oncology. This is an open access article published by Thieme under the terms of the Creative Commons Attribution-NonDerivative-NonCommercial-License, permitting copying and reproduction so long as the original work is given appropriate credit. Contents may not be used for commercial purposes, or adapted, remixed, transformed or built upon. (https://creativecommons.org/licenses/by-nc-nd/4.0/.)
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India
Abstract
Pilomatrix carcinoma is an exceedingly rare skin adnexal neoplasm derived from piliferous follicles, usually occurring in the head and neck region. Localization of this tumor in the breast is a rarity. We now report an unusual case of a 49-year-old female who presented with a palpable mass in the left breast for 2 years. Mammogram revealed a large, lobulated opacity with calcification, and positron emission tomography–computed tomography showed a metabolically active soft tissue mass measuring 15 cm involving all the quadrants of the left breast. Subsequently, the patient underwent radical mastectomy, and histopathologic diagnosis of pilomatrix carcinoma of the breast was offered. Pilomatrix carcinoma, although exceedingly rare, can have an intramammary location and can be misdiagnosed as breast carcinoma on limited material. A high index of suspicion is required to arrive at an accurate diagnosis so as to obviate neoadjuvant chemotherapy.
Introduction
Pilomatrix carcinoma is a rare malignant counterpart of pilomatrixoma, a cutaneous adnexal neoplasm with hair follicle and matrix differentiation.[1] These tumors occur more often in middle-aged men and are most frequently located in the head and neck region, followed by upper and lower extremities.[2,3] Intramammary localization of this tumor is very rare, and only an occasional case has been reported in the literature.[2] The present case demonstrates the diagnostic dilemma due to an exceedingly unusual location of pilomatrix carcinoma masquerading as breast carcinoma.
Case Report
A 49-year-old female presented elsewhere with a 2-year history of a palpable, enlarging mass in the left breast. Bilateral mammography revealed a large, lobulated opacity with chunky calcification in the left breast [Figure 1]. The right breast was unremarkable.
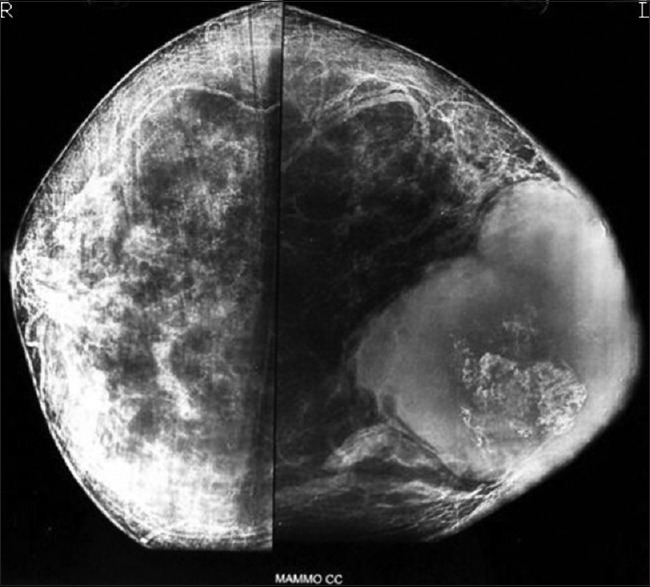
| Figure 1:Breast mammography showing a large, lobulated mass with chunky calcification in the breast
Positron emission tomography–computed tomography showed a large metabolically active soft tissue mass with necrosis and calcification involving multiple quadrants of the left breast (12.6 cm × 8.8 cm, maximum standardized uptake value 16.5). Fine-needle aspiration cytology of the left breast lump was suggestive of carcinoma, following which the patient underwent incisional biopsy. The biopsy was reported as metaplastic carcinoma elsewhere and immunohistochemistry (IHC) for estrogen receptor, progesterone receptor, and human epidermal growth factor receptor was reported to be negative.
The patient received four cycles of neoadjuvant chemotherapy (docetaxel with epirubicin) elsewhere, following which there was an increase in the size of lesion. She was then referred to our tertiary cancer institute for further management.
On physical examination, the left breast lump was hard occupying entire breast. The outside biopsy material was reviewed at our institute, and differential diagnosis of skin adnexal tumor and metaplastic carcinoma was considered. Subsequently, she underwent left radical mastectomy. Serial sectioning of the radical mastectomy specimen revealed a relatively well-circumscribed, whitish tumor measuring 15 cm × 15 cm × 10 cm involving all the quadrants of the breast with focal ulceration of the overlying skin. The tumor was firm to hard with focal areas of necrosis [Figure 2]. Microscopically, the tumor was composed of irregular islands of proliferating basaloid cells with dystrophic calcification, foci of necrosis [Figure 3a and andb],b], and with infiltrative borders [Figure 3c]. Overlying dermis was infiltrated; however, epidermis was free [Figure 3d]. The tumor had two types of epithelial cells: basaloid and ghost/mummified cells. The transition from basal to squamous areas was abrupt in most areas [Figure 3b]. The basaloid cells were irregular with scant cytoplasm and hyperchromatic nuclei. Some of which had vesicular nuclei with prominent nucleoli [Figure 3e]. Squamous differentiation was seen in the form of mummified/ghost cells and keratin masses. The ghost cells had abundant pale eosinophilic cytoplasm with faint degenerated nuclear outlines. In the actively proliferating basaloid cell area, the mitotic rate was 1–2 per high-power field [Figure 3e] with the presence of atypical mitoses. Ki-67 (MIB1) labeling index was 75% in the highest proliferating areas [Figure 3f]. There was no evidence of lymphovascular or perineural invasion. Nipple, areola, and margins of resection were uninvolved. Axillary (n = 23) and apical (n = 7) lymph nodes were free of tumor. Based on the histopathology findings, the final diagnosis of pilomatrix carcinoma was offered. The patient's postoperative course was uneventful. In view of large tumor size and high propensity of pilomatrix carcinoma for local recurrences and metastasis, the patient was treated with external beam radiotherapy (EBRT) to the left chest wall postoperatively. The patient is alive for 14 months till date without any history of recurrence or metastasis.

| Figure 2:Gross photograph of radical mastectomy showing a relatively well-circumscribed, whitish tumor involving breast with areas of calcification and focal necrosis
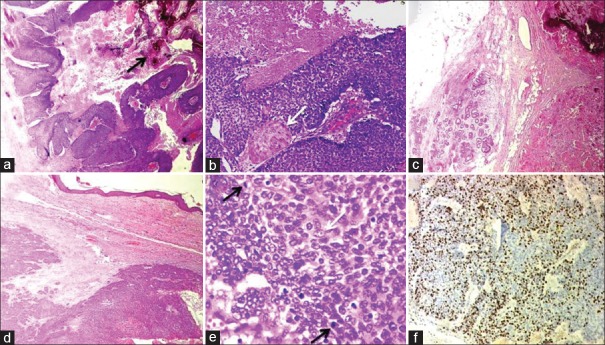
| Figure 3:H and E (a-e) and IHC (f) Photomicrographs of Pilomatrix carcinoma. (a) Islands of proliferating basaloid cells with focal necrosis and calcification (arrow) (×40). (b) Abrupt squamous differentiation (arrow) and necrosis (×100). (c) The tumor infiltrating the fat (×40). (d) Infiltrative tumor borders with desmoplastic reaction (×40). (e) Irregular basaloid cells, few with vesicular nuclei, prominent nucleoli (white arrow) and with brisk mitotic activity (black arrow) (×400). (f) Ki-67 immunostain demonstrating high proliferation index within the basaloid cells (×100)
Discussion
Pilomatrixoma, first described by Malherbe and Chenantais in 1880 as a “calcifying epithelioma,” is most frequently encountered in children and young patients as a painless, slow-growing mass predominantly involving the head and neck region. Pilomatrix carcinoma, a malignant counterpart of pilomatrixoma, is an exceedingly rare entity. The latter term was coined by Lopansri and Mihm[4] in 1980 for infiltrative tumors comprised of actively proliferating, hyperchromatic, vesicular basal cells with numerous mitosis. Since then, <150 href="https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5686986/#ref2" rid="ref2" class=" bibr popnode tag_hotlink tag_tooltip" id="__tag_649899897" role="button" aria-expanded="false" aria-haspopup="true" xss=removed>2,3]
There is an uncertainty in the literature regarding the origin of this tumor in the breast. Some studies mention its origin from the periareolar piliferous bulbs mimicking a breast malignancy.[5] Repeated skin trauma inciting inflammatory response is also considered as one of the causes of this lesion, whereas another hypothesis is that these are hamartomas.[6]
In the largest series of twenty cases of pilomatrix carcinoma to date, a single case had an intramammary location.[2] The patient was a 57-year-old female patient who presented with a nodule measuring 2.5 cm in the left breast for duration of 3 years. The nodule was excised, and the patient was alive with no history of recurrence.[2] The patient, in the present study, had a history of enlarging mass in the left breast for 2-year duration, measuring 15 cm, and the patient had to undergo modified radical mastectomy, followed by EBRT. The patient is alive till date for 14 months after the treatment completion without any history of recurrences.
On histopathological evaluation, the major diagnostic dilemma is making a distinction between pilomatrix carcinoma and relatively frequently encountered benign pilomatrixoma. Findings that distinguish pilomatrix carcinoma from pilomatrixoma include the presence of large tumor size (4 cm or more), infiltrative growth pattern, basaloid cell proliferation with cytologic atypia, necrosis, desmoplastic stroma, frequent and abnormal mitosis, and vascular, lymphatic, or perineural invasion.[7] The mitotic activity is reported to be as high as 30 or more mitoses per 10 high-power fields.[8] In the present case, the features that were noted to establish the diagnosis of pilomatrix carcinoma were large tumor size (15 cm), basaloid cell proliferation with atypia, infiltrative tumor borders with desmoplastic stroma, frequent and atypical mitoses, and areas of necrosis. On IHC, Ki-67 (MIB1) labeling index was 75% in the highest proliferating areas which is in range published previously.[8,9]
Pilomatrix carcinomas are locally aggressive tumors with an increased tendency to recur, especially in cases with incomplete excision. Higher incidences of recurrence and death were found to be associated with increased anaplasia and deep soft tissue infiltration.[2] Metastatic disease has been reported in 13% of cases and found to be significantly associated with local tumor recurrence (P < 0 href="https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5686986/#ref3" rid="ref3" class=" bibr popnode tag_hotlink tag_tooltip" id="__tag_649899891" role="button" aria-expanded="false" aria-haspopup="true" xss=removed>3] In the present case, there was no evidence of nodal or distant metastasis.
The other differential diagnoses include tumors composed of basaloid cells, viz; basal cell carcinoma and trichoepithelioma, squamous cell carcinoma, and mixed tumors of the skin.[8,10] Basal cell carcinoma is characterized by basal cell proliferation with peripheral palisading and retraction spaces separating the nests from surrounding stroma. Shadow cells and follicular differentiation are not seen in basal cell carcinoma. Trichoepithelioma is a well-circumscribed, superficial dermal lesion composed of horn cysts and basaloid epithelial formations. The basophilic cells show peripheral palisading and are surrounded by stroma with moderate number of fibroblasts. The keratinization is abrupt and complete, and shadow cells are not seen. Poorly differentiated squamous carcinoma can have basophilic appearance with greater nuclear atypia and mitotic activity.[7] In the breast, pilomatrix carcinoma may be misdiagnosed as metaplastic carcinoma on limited material, as in the present case.
Although a standard treatment protocol for pilomatrix carcinoma is lacking, gross total resection is the recommended treatment to limit tumor recurrence. RT alone or as adjuvant therapy with surgery has been used in locally advanced or metastatic cases. However, the exact role of RT is unclear due to limited data. Systemic chemotherapy has also been largely unsuccessful.[3] The patient in the present case underwent radical mastectomy with negative excision margin followed by EBRT to the left chest wall due to large tumor size and to prevent recurrence.
Conclusion
Pilomatrix carcinoma, although extremely rare, can present as an intramammary tumor mimicking as breast carcinoma. A high index of suspicion is required to arrive at an accurate diagnosis so as to obviate neoadjuvant chemotherapy. In view of high incidence of local recurrences, it is mandatory to have continued close follow-up of the patient.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- AlSharif S, Meguerditchian A, Omeroglu A, Lamarre P, Altinel G, Mesurolle B. Pilomatricoma of the male breast: Sonographic mammographic MRI features with pathologic correlation. Clin Imaging 2015;39:308-10.
- Sau P, Lupton GP, Graham JH. Pilomatrix carcinoma. Cancer 1993;71:2491-8.
- Herrmann JL, Allan A, Trapp KM, Morgan MB. Pilomatrix carcinoma: 13 new cases and review of the literature with emphasis on predictors of metastasis. J Am Acad Dermatol 2014;71:38-43.e2.
- Lopansri S, Mihm MC Jr. Pilomatrix carcinoma or calcifying epitheliocarcinoma of Malherbe: A case report and review of literature. Cancer 1980;45:2368-73.
- Nori J, Abdulcadir D, Giannotti E, Calabrese M. Pilomatrixoma of the breast, a rare lesion simulating breast cancer: A case report. J Radiol Case Rep 2013;7:43-50.
- Forbis R Jr., Helwig EB. Pilomatrixoma (calcifying epithelioma). Arch Dermatol 1961;83:606-18.
- Elder DE, Elenitsas R, Johnson BL, Murphy GF. Lever's Histopathology of the Skin. 9th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2005. p. 871-86.
- Daurte VM, Sepahdari AR, Abasolo PA, John MS. Pilomatrix carcinoma of the head and neck: Case report and review of the literature. Int J Otolaryngol Head Neck Surg 2012;1:53-6.
- Aherne NJ, Fitzpatrick DA, Gibbons D, Armstrong JG. Pilomatrix carcinoma presenting as an extra axial mass: Clinicopathological features. Diagn Pathol 2008;3:47.
- Arslan D, Gündüz S, Avci F, Merdin A, Tatli AM, Uysal M, et al. Pilomatrix carcinoma of the scalp with pulmonary metastasis: A case report of a complete response to oral endoxan and etoposide. Oncol Lett 2014;7:1959-61.

| Figure 1:Breast mammography showing a large, lobulated mass with chunky calcification in the breast

| Figure 2:Gross photograph of radical mastectomy showing a relatively well-circumscribed, whitish tumor involving breast with areas of calcification and focal necrosis

| Figure 3:H and E (a-e) and IHC (f) Photomicrographs of Pilomatrix carcinoma. (a) Islands of proliferating basaloid cells with focal necrosis and calcification (arrow) (×40). (b) Abrupt squamous differentiation (arrow) and necrosis (×100). (c) The tumor infiltrating the fat (×40). (d) Infiltrative tumor borders with desmoplastic reaction (×40). (e) Irregular basaloid cells, few with vesicular nuclei, prominent nucleoli (white arrow) and with brisk mitotic activity (black arrow) (×400). (f) Ki-67 immunostain demonstrating high proliferation index within the basaloid cells (×100)
References
- AlSharif S, Meguerditchian A, Omeroglu A, Lamarre P, Altinel G, Mesurolle B. Pilomatricoma of the male breast: Sonographic mammographic MRI features with pathologic correlation. Clin Imaging 2015;39:308-10.
- Sau P, Lupton GP, Graham JH. Pilomatrix carcinoma. Cancer 1993;71:2491-8.
- Herrmann JL, Allan A, Trapp KM, Morgan MB. Pilomatrix carcinoma: 13 new cases and review of the literature with emphasis on predictors of metastasis. J Am Acad Dermatol 2014;71:38-43.e2.
- Lopansri S, Mihm MC Jr. Pilomatrix carcinoma or calcifying epitheliocarcinoma of Malherbe: A case report and review of literature. Cancer 1980;45:2368-73.
- Nori J, Abdulcadir D, Giannotti E, Calabrese M. Pilomatrixoma of the breast, a rare lesion simulating breast cancer: A case report. J Radiol Case Rep 2013;7:43-50.
- Forbis R Jr., Helwig EB. Pilomatrixoma (calcifying epithelioma). Arch Dermatol 1961;83:606-18.
- Elder DE, Elenitsas R, Johnson BL, Murphy GF. Lever's Histopathology of the Skin. 9th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2005. p. 871-86.
- Daurte VM, Sepahdari AR, Abasolo PA, John MS. Pilomatrix carcinoma of the head and neck: Case report and review of the literature. Int J Otolaryngol Head Neck Surg 2012;1:53-6.
- Aherne NJ, Fitzpatrick DA, Gibbons D, Armstrong JG. Pilomatrix carcinoma presenting as an extra axial mass: Clinicopathological features. Diagn Pathol 2008;3:47.
- Arslan D, Gündüz S, Avci F, Merdin A, Tatli AM, Uysal M, et al. Pilomatrix carcinoma of the scalp with pulmonary metastasis: A case report of a complete response to oral endoxan and etoposide. Oncol Lett 2014;7:1959-61.


 PDF
PDF  Views
Views  Share
Share

