Plasma Epstein–Barr virus and Hepatitis B virus in non-Hodgkin lymphomas: Two lymphotropic, potentially oncogenic, latently occurring DNA viruses
CC BY-NC-ND 4.0 · Indian J Med Paediatr Oncol 2016; 37(03): 146-151
DOI: DOI: 10.4103/0971-5851.190353
Abstract
Context: There is a need to study potential infective etiologies in lymphomas. Lymphocyte-transforming viruses can directly infect lymphocytes, disrupt normal cell functions, and promote cell division. Epstein–Barr virus (EBV) is known to be associated with several lymphomas, especially Hodgkin lymphomas (HLs). And recently, the lymphocyte-transforming role of hepatitis B virus (HBV) has been emphasized. Aims: The aim of this study was to elucidate the association of two potentially oncogenic, widely prevalent latent DNA viruses, EBV and HBV, in non-HL (NHL). Settings and Design: In this prospective study, we estimated plasma EBV and HBV DNA in NHL patients. Materials and Methods: Peripheral blood was obtained from newly diagnosed, treatment na ïve, histologically confirmed NHL patients. Plasma EBV DNA was quantified by real-time polymerase chain reaction (PCR) targeting Epstein–Barr Nucleic acid 1 while the plasma HBV DNA was detected using nested PCR targeting HBX gene. In a small subset of patients, follow-up plasma samples post-anticancer chemotherapy were available and retested for viral DNA. Results: Of the 110 NHL patients, ~79% were B-cell NHL and ~21% were T-cell NHL. Plasma EBV-DNA was detected in 10% NHLs with a higher EBV association in Burkitt lymphoma (33.3%) than other subtypes. Pretherapy HBV DNA was detected in 21% NHLs; most of them being diffuse large B-cell lymphoma (DLBCL). Moreover, 42% of DLBCL patients had HBV DNA in plasma. Since all patients were HBV surface antigen seronegative at diagnosis, baseline plasma HBV-DNAemia before chemotherapy was indicative of occult hepatitis B infection. Conclusions: Our findings indicate a significant association of HBV with newly diagnosed DLBCL.
Keywords
Diffuse large B-cell lymphoma - Epstein–Barr virus - hepatitis B virus - non-Hodgkin lymphoma - occult hepatitis B virus infectionPublication History
Article published online:
12 July 2021
© 2016. Indian Society of Medical and Paediatric Oncology. This is an open access article published by Thieme under the terms of the Creative Commons Attribution-NonDerivative-NonCommercial-License, permitting copying and reproduction so long as the original work is given appropriate credit. Contents may not be used for commercial purposes, or adapted, remixed, transformed or built upon. (https://creativecommons.org/licenses/by-nc-nd/4.0/.)
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India
Abstract
Context:
There is a need to study potential infective etiologies in lymphomas. Lymphocyte-transforming viruses can directly infect lymphocytes, disrupt normal cell functions, and promote cell division. Epstein–Barr virus (EBV) is known to be associated with several lymphomas, especially Hodgkin lymphomas (HLs). And recently, the lymphocyte-transforming role of hepatitis B virus (HBV) has been emphasized.
Aims:
The aim of this study was to elucidate the association of two potentially oncogenic, widely prevalent latent DNA viruses, EBV and HBV, in non-HL (NHL).
Settings and Design:
In this prospective study, we estimated plasma EBV and HBV DNA in NHL patients.
Materials and Methods:
Peripheral blood was obtained from newly diagnosed, treatment na ïve, histologically confirmed NHL patients. Plasma EBV DNA was quantified by real-time polymerase chain reaction (PCR) targeting Epstein–Barr Nucleic acid 1 while the plasma HBV DNA was detected using nested PCR targeting HBX gene. In a small subset of patients, follow-up plasma samples post-anticancer chemotherapy were available and retested for viral DNA.
Results:
Of the 110 NHL patients, ~79% were B-cell NHL and ~21% were T-cell NHL. Plasma EBV-DNA was detected in 10% NHLs with a higher EBV association in Burkitt lymphoma (33.3%) than other subtypes. Pretherapy HBV DNA was detected in 21% NHLs; most of them being diffuse large B-cell lymphoma (DLBCL). Moreover, 42% of DLBCL patients had HBV DNA in plasma. Since all patients were HBV surface antigen seronegative at diagnosis, baseline plasma HBV-DNAemia before chemotherapy was indicative of occult hepatitis B infection.
Conclusions:
Our findings indicate a significant association of HBV with newly diagnosed DLBCL.
INTRODUCTION
Non-Hodgkin lymphomas (NHL) include a heterogeneous group of lymphomas of B- or T-cell origin, which are either aggressive or indolent in nature.[1] It is well known that lymphocyte-transforming viruses can directly infect lymphocytes, disrupt normal cell functions, and promote cell division.[1]
Epstein–Barr virus (EBV) is a γ-herpesvirus that infects more than 95% of the adult population worldwide.[2] It is known to be associated, probably even causally, with several lymphomas in innumerable studies spanning decades.[2] EBV is usually acquired in early childhood and thereafter persists lifelong in the host in a latent state in memory B lymphocytes.[2] EBV is associated with several benign and neoplastic diseases. Nasopharyngeal carcinomas and posttransplant lymphoproliferative disorders are nearly always EBV-associated; Hodgkin's lymphoma (HL) is associated with EBV in 40–50% while NHL other than Burkitt's lymphoma and gastric adenocarcinomas is less uniformly EBV-associated.[2]
On the other hand, hepatitis B virus (HBV), a member of the family Hepadnaviridae, infects nearly 350 million people worldwide and causes nearly 340,000 liver cancer cases annually.[3] The lymphocyte-transforming role of HBV has recently come to the forefront with several studies over the last decade.[4] Occult hepatitis B infection (OBI) is a hitherto underestimated, but currently, a well-recognized entity worldwide with HBV persisting latently in hepatocytes and lymphocytes.[5] Once infected, complete eradication of HBV is rare and most often a replication-competent HBV DNA persists in the liver or lymphocytes or both for many years or even life.[6]
Studies from India on viral association with lymphomas are few. The aim of this study was to determine the association of plasma EBV and HBV DNA in newly diagnosed, treatment naïve, human immunodeficiency virus (HIV) seronegative NHL patients.
MATERIALS AND METHODS
The study was carried out prospectively at a Regional Cancer Center in South India. The study was approved by the Institutional Medical Ethics Committee and was performed in accordance with the Helsinki Declaration 2000. Informed consent was obtained from all patients before enrollment in the study.
Study population
The target population included patients with a diagnosis of NHL following histopathological examination of diagnostic biopsies of the involved lymph node. The final diagnosis was confirmed by immunohistochemistry in all cases included in the study; CD3+ denoting T-cell subtype and CD20+ denoting B-cell subtype. To help enroll relatively immunocompetent patients and minimize the possibility of viral reactivation, we excluded lymphoma patients who were seroreactive to HIV.
NHL group was classified according to the WHO 2008 Classification and staging was done by the Ann Arbor staging system. In the majority of patients, four to six cycles of a standard cyclophosphamide, adriamycin, vincristine, prednisolone (CHOP) regimen with or without rituximab and regional radiation therapy to the involved body regions were given to early-stage patients (Stage I or II). Six to eight cycles of CHOP/rituximab-CHOP or more aggressive CHOP-based regimens were given to patients with advanced-stage disease or with early-stage, bulky disease.
Sample collection
In all cases, a peripheral blood sample was collected before initiation of anticancer chemotherapy. Postchemotherapy, follow-up blood sample could be obtained in a small subset of patients (n = 20). In these patients, response to treatment and outcome were recorded. Plasma was separated from all blood samples soon after collection by centrifugation at 1200 g for 2 min and stored frozen at −20°C until DNA extraction. All samples were handled with sterile precautions using aerosol-free tips; DNA was extracted from 250 µl of plasma by a silica-based manual extraction protocol, eluting with 50 µl water.
Plasma Epstein–Barr virus polymerase chain reaction
Plasma EBV is used as a surrogate marker of EBV-associated lymphomas and has been validated in our earlier studies on childhood onset [7] and adult onset HLs (unpublished data) using EBV latent membrane protein-1 immunohistochemistry as the standard.[2] In this study, plasma EBV DNA was quantified by real-time polymerase chain reaction (PCR) targeting the Epstein–Barr nucleic acid 1 region of the virus as per previously published protocol.[7,8] The assay was validated using serial 10-fold dilutions of standard EBV DNA (a kind gift from Professor Y.L. Kwong, University Department of Medicine, Queen Mary Hospital, Hong Kong). EBV DNA load >500 copies/ml was considered EBV-positive similar to current studies.[9]
Plasma hepatitis B virus polymerase chain reaction
Detection of HBV DNA in serum/plasma is used as one of the methods for detecting OBI and has been used for establishing HBV association with malignancies such as hepatocellular carcinoma and NHL.[10,11,12] In this study, HBV DNA was detected using a nested PCR method targeting a conserved region of the HBX gene.[11] DNA extracted from plasma of an hepatitis B surface antigen (HBsAg) seropositive patient acted as an in-house positive control. We chose to detect the HBX gene (and not the HBS gene) due to its pro-oncogenicity.[10]
Statistical correlation
Fisher's exact test (two-tailed) was used to measure significance of association of viral DNA with the different subtypes of NHL. P < 0.05 was considered statistically significant.
RESULTS
Over a period of 10 months, 156 histologically confirmed cases of lymphomas were diagnosed, of which 110 were diagnosed as NHL; 77 males, 33 females; 97 adults, 13 pediatric (mean age of 44.7 ± 18.7 years; range 4–81 years). The predominant subtype was diffuse large B-cell lymphoma (DLBCL) accounting for 43.6% of NHLs, followed by follicular lymphoma (14.5%), peripheral T-cell lymphoma (10%), and Burkitt lymphoma (8.2%) [Table 1]. All patients enrolled in the study were HBsAg seronegative.
Table 1
Classification of non-Hodgkin lymphoma with pretherapy plasma Epstein–Barr virus and hepatitis B virus status
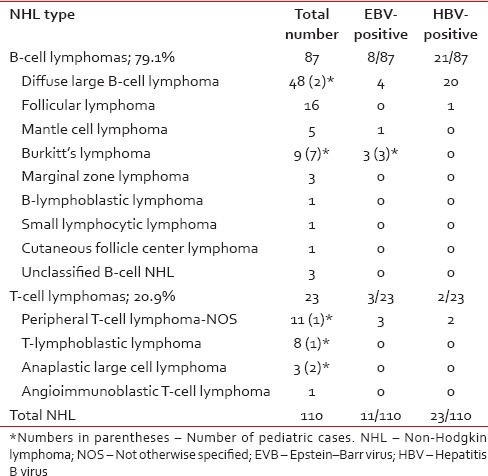
Plasma EBV DNA was detectable in 11 patients: 8 B-NHLs, 3 T-NHLs. Among subtypes with high EBV positivity were Burkitt lymphoma (33.3%) followed by peripheral T-cell lymphoma and DLBCL. The EBV DNA load of the samples ranged from 1.1 × 103 to 2.5 × 106 copies/ml (mean 296.6 × 103 copies/ml).
Despite all patients being HBsAg seronegative at diagnosis, plasma HBV was detectable in 23 patients: 21 B-NHLs, 2 T-NHLs. Over 95% of these HBV-positive B-NHL were of DLBCL subtype (20/21); however overall, ~42% of DLBCLs were HBV-positive (20/48). Fisher's exact test showed a significant association of HBV with the NHL subtype, DLBCL (P < 0.0001). Amplified products of HBX gene from ten of these DLBCL cases were sequenced using the Sanger dideoxy sequencing method. Sequencing reactions were completed using the BigDye Terminator Chemistry version 3.1 (ABI 3130XL Genetic Analyzer/ABI 3730XL Genetic Analyzer of Applied Biosystems). All ten sequences showed 99% identity with and over 90% query coverage to HBV.
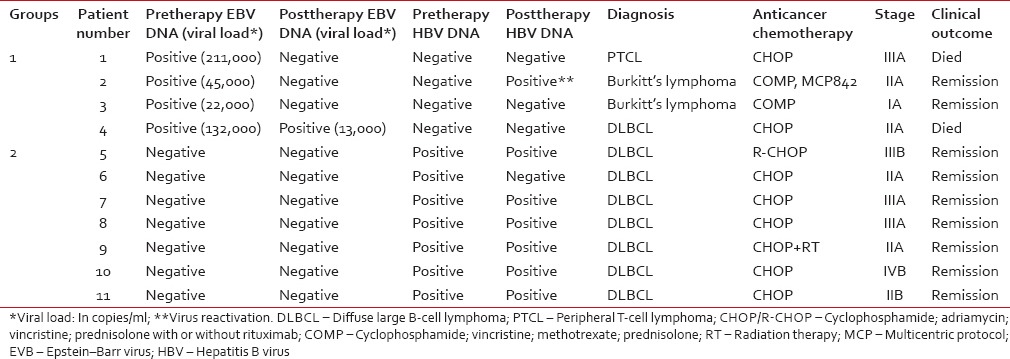
In only 20 of the 110 NHL patients, postchemotherapy follow-up blood samples were available in addition to pretherapy samples [Table 2]. Of these 20 patients, viral DNA was detectable in 11 of the pretherapy samples: 4 EBV-positive (Group 1) and 7 HBV-positive (Group 2). In the remaining 9 patients, viral DNA was undetectable. In Group 1, EBV load postchemotherapy reverted to negative in three patients [one of whom died; Patient 1, Table 2]. The fourth patient, in whom EBV DNA load postchemotherapy reduced nearly tenfold, but persisted, died after third chemotherapy cycle [Patient 4, Table 2]. In Group 2 (HBV-positive), all seven patients remained seronegative for HBsAg postchemotherapy and had clinical remission after 3–6 cycles of chemotherapy despite viral DNA persisting in six of them. In Patient 2 (Group 1, EBV-positive), pretherapy HBV DNA status changed from negative to positive postchemotherapy, indicating HBV reactivation (was clinically diagnosed as having acute flare hepatitis and was treated with lamivudine, 50 mg daily).
Table 2
Plasma viral DNAemia following anticancer treatment
DISCUSSION
Worldwide, NHL accounts for 60–80% of malignant lymphomas, 80–90% of them being B-cell NHLs.[13,14] This study revealed 70% of the lymphomas to be NHL, of which ~79% were B-cell NHLs. In accordance with other studies, the predominant NHL subtype was DLBCL, followed by follicular lymphoma, peripheral T-cell lymphoma, and Burkitt lymphoma.[14,15]
Viral infections are known to induce lymphomagenesis either inherently by transforming lymphocytes, by inducing immunodeficiency or by inducing chronic immune stimulation and persistent activation of lymphocytes.[1] Several lymphocyte-transforming viruses such as EBV, HBV, human herpes virus 8, human T-lymphotropic virus 1, HIV, and hepatitis C virus that have been found associated with NHLs.[1,4,16] Due to their increased prevalence, ability to remain latent, oncogenicity, and lymphotropism, EBV and HBV were studied in NHLs in this study.
NHLs are a heterogeneous group of lymphomas with EBV association varying according to geographical location, race, age, subtype, or histological characteristics.[2,17] Unlike HL, which is more uniformly EBV-associated (overall ~40%), NHL shows a varied and lower association (overall ~5%), except when it is acquired immunodeficiency syndrome-related NHL (overall ~40%).[2] High EBV association is seen in certain NHL subtypes such as nasal natural killer cell lymphomas and endemic Burkitt lymphoma.[2] Studies exploring the EBV association with NHL are numbered and use different tools to determine EBV association: Epstein–Barr virus-encoded small RNA (EBER) in situ hybridization,[18,19] immunohistochemistry, EBV PCR,[18] and quantitative real-time PCR.[8,20] Using quantitative PCR, we found plasma EBV DNA in an overall 10% of NHLs. Subtypes with relatively high EBV association were Burkitt lymphoma (33.3%), peripheral T-cell lymphoma, and DLBCL. Although EBV association ranges from >95% in endemic Burkitt lymphoma to ~20% in sporadic Burkitt lymphoma, in developing countries like India, it is “intermediate” between sporadic and endemic types and varies from 25% to 80%.[19] An earlier collaborative study from our institute, using EBER In-situ hybridization on lymph node sections, had shown an EBV association in 80% of Burkitt lymphomas from India and 47% of Burkitt lymphomas from Argentina.[19]
In recent years, several large case–control studies have demonstrated a significant association between HBV infection and NHLs with the odds of HBV infection of 2.5 in NHL patients [12,21,22] and similar findings from cohort studies on HBV-infected and noninfected cases.[3,23] The study from South Korea demonstrated 12.8% HBsAg seropositivity among NHLs,[3] and a meta-analytic study revealed overt HBV infection to range from 4.9% to 27.3%.[24] However, in these studies, OBI was not detected. Occult HBV infection, indicating low-level viral replication, probably plays a significant role in lymphomagenesis and may be underestimated in serology-based studies.[6] Our study on HBsAg-negative patients demonstrated plasma HBV DNA (X gene) in ~21% of NHLs. Ideally, detecting the gene in lymphoma tissue would reveal the true association between the two. A study from Egypt detected HBV DNA in 27.5% of tumor tissue (involved lymph nodes in lymphoma patients) but not in normal lymph nodes (normal nodes from other cancers).[25]
In the present study, 87% of the HBV-associated NHLs were of DLBCL subtype. This is in accordance with many studies that have found a greater association of HBV with DLBCL subtypes, than with other NHLs.[3,12,26] In the South Korean cohort study, 14.5% of DLBCLs were HBsAg-seropositive.[3] Similar to our findings, a study from Taiwan, using nested PCR to detect HBV-DNA in patients’ sera, found a 23.5% incidence of HBV infection in B-NHLs (especially in DLBCL) and a high prevalence of occult HBV infection in HBsAg-negative B-NHL.[12] Wang et al. demonstrated higher HBV DNA positivity in B-NHL (predominantly DLBCL) than in T-NHL and other lymphoproliferative disorders in DNA extracted from paraffin-embedded nodes (38.3% vs. 11.8%) and fresh lymph nodes (55% vs. 15.4%), respectively, suggesting that chronic HBV infection in lymph nodes is likely to be associated with B-cell lymphoma.[26] Most of these studies are from the Asian subcontinent, mainly the Far East, and have a bearing with the high endemicity of HBV in that geographical region.
Since all patients included in this study were HBsAg-seronegative, HBV DNA positivity in 21% NHLs indicates OBI with possible viral replication occurring within the host. Occult HBV infection is underestimated not only in India but also worldwide.[5,27] In an Indian study involving healthy blood donors, the prevalence of antiHBc antibodies was reported to be 30%; about one third of whom harbored the viral DNA indicative of OBI.[27] In a recent study on newly diagnosed acute lymphocytic leukemic pediatric patients, we found antiHBc antibodies in 39%; however, since HBV DNA was not tested, we could not ascertain the true OBI rates.[28]
In this study, we have attempted to elucidate association of EBV and HBV in NHL patients. Considering the increased importance of HBV with DLBCL, our study becomes all the more relevant. However, there were a few limitations in our study. Use of end-point PCR for HBV and not quantitative PCR precluded viral load estimation for HBV. Dismal follow-up sample acquisition postchemotherapy resulted in inability to assess plasma EBV load as a prognostic marker for therapeutic responsiveness through the course of chemotherapy. While EBV DNA reverted to negative in three patients postchemotherapy, it reduced, but was still detectable in one patient. On the other hand, HBV DNA reverted to negative postchemotherapy in only one out of seven patients despite all patients showing clinical remission. There could be two reasons for this. Considering HBV-associated DLBCL occurs in the background of OBI, the virus is produced not only in the hepatocytes but also by transformed lymphoid cells. In patients with clinical remission, cytotoxic chemotherapy is able to target only one source of the virus, namely, HBV bearing lymphoma cells, but probably not the hepatocytes – where the virus may continue to be produced and be intermittently released into the bloodstream. Alternatively, there could have been a decrease in HBV DNA load postchemotherapy, which could not be ascertained as we used end-point PCR and not quantitative PCR. Overall, in HBV associated lymphomas, use of circulating viral DNA as a surrogate prognosticating marker of therapeutic response may not be of clinical utility unlike in EBV-associated lymphomas.
CONCLUSIONS
Our study revealed cell-free EBV association in 10% and HBV DNA association in 21% of treatment naïve, HIV-negative NHL patients. Since all patients were HBsAg-seronegative, the presence of HBV DNA in plasma indicates occult HBV infection in those patients. While difference in EBV association between the B-cell NHL and T-cell NHL was not significant, there was a trend toward association of HBV with the former. Among NHL subtypes, there was a significant association of HBV with DLBCL. There are very few studies from India on viral association in NHLs. Ease of sampling and performance of the plasma viral DNA estimation render it a simple and useful tool for detecting EBV or HBV association in lymphomas. There is a scope for large scale studies in future, with active postchemotherapy follow-up in virus-positive lymphomas. In future studies, the possible etiological role of HBV, especially occult HBV infection, needs to be ratified by investigating localization of the viral DNA in tumor cells of these lymphomas.
Financial support and sponsorship
The study was funded by a research grant from Rajiv Gandhi University of Health Sciences, Bengaluru to M. Sinha. The funding source had no involvement in study design, collection, analysis, and interpretation of data, in writing the report and in the decision to submit the article for publication.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
We wish to gratefully acknowledge the generous gift of quantified EBV DNA by Professor Y.L. Kwong, University Department of Medicine, Queen Mary Hospital, Hong Kong.


 PDF
PDF  Views
Views  Share
Share

