Predictors of Survival in Children with Osteogenic Sarcoma Undergoing Limb Salvage Surgery: Experience from a Tertiary Cancer Center in Rural India
CC BY-NC-ND 4.0 · Indian J Med Paediatr Oncol 2020; 41(03): 335-339
DOI: DOI: 10.4103/ijmpo.ijmpo_166_18
Abstract
Context: Osteogenic Sarcoma (OGS) is the fifth most common malignancy among adolescents aged 15–19. With multimodality therapy, the long-term survival has improved from 16% in the prechemotherapy era to around 70% in the postchemotherapy era. Aim: This study aims to determine the clinical profile and survival of children with OGS being treated with limb-salvage surgery (LSS). Subjects and Methods: This is a retrospective analysis of all cases of OGS (age ≤ 19) who underwent LSS at our center between June 2009 and February 2017. Baseline characteristics were noted and multivariate analysis was performed for various variables to identify predictors of survival. Results: Among 44 cases studied majority were boys (n = 27). Ninety-three percentage (n = 41) were adolescents. Stage 2 disease was 75% and Stage 3 disease was 25%. The estimated 3-year overall survival (OS) was 69% (95% confidence interval [CI] 55–86) and the estimated 3-year event-free survival (EFS) was 55% (95% CI = 41–74). OS was significantly improved in patients with >90% necrosis postneoadjuvant chemotherapy (NACT) when compared with <90% necrosis (3-year OS = 88% vs. 51%,P= 0.01) and in patients who received ≤4 cycles NACT versus >4 cycles (78% vs. 60%,P= 0.04). EFS was significantly better in patients without lung metastasis at presentation (61% vs. 29%,P= 0.04), Stage 2 disease (59% vs. 38%,P= 0.04) and >90% necrosis in the tumor post-NACT (69% vs. 35%,P= 0.02). Conclusion: Significant response to NACT predicted improved OS and EFS in children with OGS treated with LSS.
Publication History
Received: 26 July 2018
Accepted: 02 January 2020
Article published online:
28 June 2021
© 2020. Indian Society of Medical and Paediatric Oncology. This is an open access article published by Thieme under the terms of the Creative Commons Attribution-NonDerivative-NonCommercial-License, permitting copying and reproduction so long as the original work is given appropriate credit. Contents may not be used for commercial purposes, or adapted, remixed, transformed or built upon. (https://creativecommons.org/licenses/by-nc-nd/4.0/.)
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India
Abstract
Context: Osteogenic Sarcoma (OGS) is the fifth most common malignancy among adolescents aged 15–19. With multimodality therapy, the long-term survival has improved from 16% in the prechemotherapy era to around 70% in the postchemotherapy era. Aim: This study aims to determine the clinical profile and survival of children with OGS being treated with limb-salvage surgery (LSS). Subjects and Methods: This is a retrospective analysis of all cases of OGS (age ≤ 19) who underwent LSS at our center between June 2009 and February 2017. Baseline characteristics were noted and multivariate analysis was performed for various variables to identify predictors of survival. Results: Among 44 cases studied majority were boys (n = 27). Ninety-three percentage (n = 41) were adolescents. Stage 2 disease was 75% and Stage 3 disease was 25%. The estimated 3-year overall survival (OS) was 69% (95% confidence interval [CI] 55–86) and the estimated 3-year event-free survival (EFS) was 55% (95% CI = 41–74). OS was significantly improved in patients with >90% necrosis postneoadjuvant chemotherapy (NACT) when compared with <90% necrosis (3-year OS = 88% vs. 51%,P= 0.01) and in patients who received ≤4 cycles NACT versus >4 cycles (78% vs. 60%,P= 0.04). EFS was significantly better in patients without lung metastasis at presentation (61% vs. 29%,P= 0.04), Stage 2 disease (59% vs. 38%,P= 0.04) and >90% necrosis in the tumor post-NACT (69% vs. 35%,P= 0.02). Conclusion: Significant response to NACT predicted improved OS and EFS in children with OGS treated with LSS.
Keywords
Limb salvage surgery - osteosarcoma - prognostic factorsIntroduction
Osteogenic sarcoma (OGS) is the most frequent primary malignant bone tumor in children and adolescents.[1] With the addition of chemotherapy, the long-term survival in this group of patients has improved from 16% to 70%.[2] For bone sarcomas, wide local excision of the lesion either by amputaion or a limb sparing procedure is the recommended surgical approach advised by the Musculoskeletal Tumor Society.[3],[4] For lesions involving either the upper or lower extremity, limb salvage can improve functional outcome without sacrificing local disease control as long as complete tumor resection is anatomically possible and adjuvant chemotherapy is used.[5],[6],[7],[8] Studies on children with OGS undergoing limb salvage surgery (LSS) in low middle-income countries are limited. Hence, we aim to determine the clinical profile and survival of children being treated with this modality.
Subjects and Methods
This is a retrospective analysis of all cases of OGS (age ≤19) who underwent LSS at our center between June 2009 and February 2017. After institutional review board approval, the case records were retrieved. Files in which data were incomplete were excluded.
In our institution, when a child with suspected extremity osteosarcoma comes for a workup, J needle biopsy is done after radiological investigations. Metastatic workup involves computed tomography chest and bone scan. After confirming the diagnosis, measurements are taken for custom made titanium mega prosthesis. Neoadjuvant chemotherapy (NACT) is given (IAP regimen-ifosfamide – 1.2 g/m 2 × 3 days, adriamycin – 40 mg/m 2 × 1 day, and cisplatin – 50 mg/m 2 × 2 days). After 3–4 cycles of chemotherapy, LSS is done. Remaining cycles of chemotherapy are given once surgical wound heals.
From the case files, demographic characteristics, site of tumor, treatment given, number of cycles of NACT, date of progression, date of death, and date of the last follow-up were noted. Those who did not have to follow-up till date were updated through telephonic inquiry. As per our IRB/Independent Ethics Committee (DCG (I) Reg. No: ECR/780/Inst./KL/2015/RR-19), a waiver was obtained with regard to obtaining separate consent from each subject. Details of the patient's current status were collected from parents by phone, after getting verbal consent from them.
Statistical analysis was done with SPSS Statistics for windows, Version 20.0 Armonk, NY: IBM Corp. and R i386. Survival was analyzed by Kaplan–Meier curves. Univariate analyses were performed for the variables – age class (<10 years vs. 10–19 years), sex, lung metastasis at presentation, stage of disease, post-NACT necrosis (>90% vs. <90%), and number of cycles of NACT.
Results
During the study, 53 children with OGS were registered. Nine children were excluded from the study, as one child had OGS of the skull, two children with extremity OGS underwent amputation, and accurate data were unavailable in six cases where LSS was done. Hence, a total of 44 children (≤19 years) who underwent LSS for extremity OGS were included in the study. Of 44 children, one had a pathological fracture at presentation. Details regarding the presence or absence of fracture at presentation were unavailable for six children. The remaining 37 children did not have a pathological fracture.
Reconstruction was done with custom made titanium prosthesis in all children except two (one child underwent an autograft-reconstruction with free fibular flap and one underwent extracorporeal irradiation). The median follow-up was 51 months. All of them received six cycles of IAP chemotherapy. The baseline characteristics of the sample population are shown in [Table 1]. Postoperative morbidity was present in 18% (8/44) - foot drop (9%), wound infection (9%), and deep vein thrombosis (2%). The estimated 3-year overall survival (OS) was 69% (95% confidence interval [CI] - 55–86) and the estimated 3-year event-free survival (EFS) was 55% (95% CI = 41–74) [Graph 1] and [Graph 2].
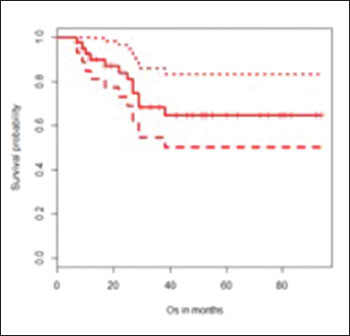
| Graph 1: Estimated 3-year overall survival
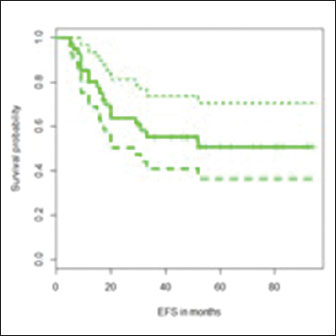
| Graph 2: Estimated 3-year event-free survival
Table 1 Baseline characteristics
OS was significantly improved in patients with >90% necrosis post-NACT when compared with <90% necrosis (3-year OS = 88% vs. 51%, P = 0.01) and in patients who received ≤4 cycles NACT versus >4 cycles (78% vs. 60%, P = 0.04) in univariate analysis. In multivariate analysis, post-NACT necrosis was the only significant predictor of OS (P = 0.03). EFS was significantly better in patients without lung metastasis at presentation (61% vs. 29%, P = 0.04), Stage 2 disease (59% vs. 38%, P = 0.04) and >90% necrosis in the tumor post-NACT (69% vs. 35%, P = 0.02) in univariate analysis. The estimated 3-year OS and EFS for nonmetastatic (Stage 2) and metastatic (Stage 3) cases were 75% versus 37% (P = 0.055) and 59% versus 38% (P = 0.04%), respectively. The results of the univariate analysis are shown in [Table 2] and [Table 3].{Table 2}{Table 3}
Predictors of overall survival
|
Factors |
Estimated 3-year OS (%) |
P |
|---|---|---|
|
OS – Overall survival; MSTS – Musculoskeletal Tumor Society; NACT – Neoadjuvant chemotherapy |
||
|
Post-NACT necrosis |
||
|
≥90% |
88 |
0.01 |
|
less than 90% |
51 |
|
|
NACT cycles |
||
|
≤4 |
78 |
0.04 |
|
less than 4 |
60 |
|
|
Age group (years) |
||
|
less than 10 |
67 |
0.91 |
|
10-19 |
68 |
|
|
Sex |
||
|
Male |
57 |
0.225 |
|
Female |
85 |
|
|
Stage (MSTS) |
||
|
2 |
75 |
0.055 |
|
3 |
37 |
|
|
Lung metastasis |
||
|
Present |
47 |
0.185 |
|
Absent |
73 |
|
Predictors of event-free survival
|
Estimated 3-year EFS (%) |
P |
|
|---|---|---|
|
EFS – Event-free survival; MSTS – Musculoskeletal Tumor Society; NACT – Neoadjuvant chemotherapy |
||
|
Post-NACT necrosis |
||
|
≥90% |
69 |
0.02 |
|
less than 90% |
35 |
|
|
NACT cycles |
||
|
≤4 |
60 |
0.078 |
|
>4 |
27 |
|
|
Age group (years) |
||
|
less than 10 |
67 |
0.772 |
|
10-19 |
54 |
|
|
Sex |
||
|
Male |
46 |
0.075 |
|
Female |
70 |
|
|
Stage (MSTS) |
||
|
2 |
59 |
0.04 |
|
3 |
38 |
|
|
Lung metastasis |
||
|
Present |
29 |
0.04 |
|
Absent |
61 |
|
Discussion
Our results emphasize that, in spite of being in a resource-limited rural tertiary cancer center, we could achieve good outcomes in children with osteosarcoma, which is comparable to international standards. A report by Sukumaran et al., which was a study done at a tertiary cancer center in a similar population, has shown 3 years' OS of 54.6% ±7.8% and disease-free survival of 43.4% ± 7.9%, which is comparable to our results. They analyzed 40 children <14 years, who underwent LSS and used the same chemotherapy regimen as in our study.[9]
An interesting observation in our study was that even though our patients received nonmethotrexate containing chemotherapy, the OS and EFS are comparable to those who received methotrexate containing regimens. Methotrexate-based regimens are the standard of care in Europe and North America.[10] In the last two decades, evidence from some countries shows that nonmethotrexate-based regimens can also yield comparable outcomes similar to our study.[11],[12],[13],[14] Recent data from Mumbai also show comparable results with nonmethotrexate-based regimen; the 5-year EFS and OS were 56% and 75%, respectively, on using cisplatin, doxorubicin, and ifosfamide.[15] In the high-income countries, the efficacy of nonmethotrexate based regimens was demonstrated in the OS99 trial.[16] However, there has not been a head-to-head comparison between three-drug regimens with and without methotrexate so far.
In a retrospective analysis of bone tumors from a tertiary center in North India, among 102 cases of OGS diagnosed in 10 years, 28 underwent LSS.[17] Elevated serum alkaline phosphatase (ALP) and number of metastasis >3 were predictive of lower EFS whereas elevated serum ALP, number of metastasis >3, and margin positivity were predictive of lower OS.[17] In a study by Bajpai et al., from Tata Memorial Hospital, Mumbai, which analyzed 100 cases of high-grade extremity-based OGS, a significant association between grade of tumor necrosis and clinical outcome was noted.[15] Response to tumor necrosis as a prognosis factor has been demonstrated in various other studies from worldwide.[18],[19],[20],[21]
The number of cycles of NACT as a prognostic factor was not demonstrated in previous studies. In our study, only 10 patients (24%) received more than four NACT cycles. The reason for the delay in surgery was either inadequate response to chemotherapy mainly because of large volume of disease or delay in procuring custom made titanium prosthesis. In this group, there was significantly reduced OS. This shows that there would be no benefit in continuing chemotherapy, to obtain more tumor control. Timely local control of the disease is a major prognostic factor as per this finding. Ours being a standalone center with limited support from other institutions, there were difficulties in minimizing the delay in procuring prosthesis.
The low sample size was a limitation of our study. Some of the patients who underwent LSS at our center took initial chemotherapy at a different center. This was also a major limitation in collecting data.
Conclusion
A significant response to NACT predicted improved OS and EFS in children with OGS treated with LSS. Those who received four NACT cycles or less showed better OS. Children who did not have lung metastasis at presentation and those who had Stage 2 disease had better EFS than others. OS in localized extremity OGS who received nonmethotrexate-based chemotherapy was comparable to that of studies from low- and middle-income countries who received methotrexate-based chemotherapy.
Acknowledgment
Sincere thanks to the Department of Cancer Registry, Malabar Cancer Centre for helping with data collection and to Dr. Chandran. K. Nair (H.O.D, Department of Clinical Hematology and Medical Oncology) for helping with statistical analysis and editing the manuscript.
Conflict of Interest
There are no conflicts of interest.
References
- Ries LA, Smith MA, Gurney JG, Linet M, Tamra T, Young JL. et al.editors. Cancer. Incidence and Survival among Children and Adolescents: United States SEER Program 1975-1995. Bethesda, MD: National Cancer Institute, SEER Program. NIH Pub.No.99-4649; 1999
- Anninga JK, Gelderblom H, Fiocco M, Kroep JR, Taminiau AH, Hogendoorn PC. et al. Chemotherapeutic adjuvant treatment for osteosarcoma: Where do we stand?. Eur J Cancer 2011; 47: 2431-45
- Peabody TD, Gibbs CPJr, Simon MA. Evaluation and staging of musculoskeletal neoplasms. J Bone Joint Surg Am 1998; 80: 1204-18
- eking WF. A system for evaluation of the surgical management of musculoskeletal tumors. In: Enneking WF. editors Limb Salvage in Musculoskeletal Oncology. New York: Churchill Livingstone; 1987. p. 145
- Goorin AM, Schwartzentruber DJ, Devidas M, Gebhardt MC, Ayala AG, Harris MB. et al. Presurgical chemotherapy compared with immediate surgery and adjuvant chemotherapy for nonmetastatic osteosarcoma: Pediatric Oncology Group Study POG-8651. J Clin Oncol 2003; 21: 1574-80
- Bacci G, Forni C, Longhi A, Ferrari S, Mercuri M, Bertoni F. et al. Local recurrence and local control of non-metastatic osteosarcoma of the extremities: A 27-year experience in a single institution. J Surg Oncol 2007; 96: 118-23
- mer RJ, Taminiau AM, Cannon SR. et al. Surgical Subcommitte of the European Osteosarcoma Intergroup. Surgical outcomes in osteosarcoma. J Bone Joint Surg Br 2002; 84: 395-400
- Wittig JC, Bickels J, Kellar-Graney KL, Kim FH, Malawer MM. Osteosarcoma of the proximal humerus: Long-term results with limb-sparing surgery. Clin Orthop Relat Res 2002; 397: 156-76
- Sukumaran RK, Rajeshwari B, Sugath S, Chellappan SG, Thankamony P, Parukuttyamma K. Methotrexate free chemotherapy and limb salvage surgery for paediatric osteosarcoma in India. Indian J Orthop 2018; 52: 58-64
- Isakoff MS, Bielack SS, Meltzer P, Gorlick R. Osteosarcoma: Current treatment and a collaborative pathway to success. J Clin Oncol 2015; 33: 3029-35
- Patel SJ, Lynch JWJr, Johnson T, Carroll RR, Schumacher C, Spanier S. et al Dose-intense ifosfamide/doxorubicin/cisplatin based chemotherapy for osteosarcoma in adults. Am J Clin Oncol 2002; 25: 489-95
- Piperno-Neumann S, Bui B, Blay J, Roché H, Pichon F, Peny A. et al. A multicentric prospective study of intensive induction chemotherapy (API-AI) in localized osteosarcoma patients: Results of a phase II trial coordinated by the French Sarcoma Group (FSG) and the FNCLCC BECT. J Clin Oncol 2006; 24 Suppl 18: 9521
- Tunn PU, Reichardt P. Chemotherapy for osteosarcoma without high-dose methotrexate: A 12-year follow-up on 53 patients. Onkologie 2007; 30: 228-32
- Assi H, Missenard G, Terrier P, Le PechouxC, Bonvalot S, Vanel D. et al. Intensive induction chemotherapy without methotrexate in adult patients with localized osteosarcoma: Results of the Institut Gustave-Roussy phase II trial. Curr Oncol 2010; 17: 23-31
- Bajpai J, Chandrasekharan A, Talreja V, Simha V, Chandrakanth MV, Rekhi B. et al. Outcomes in non-metastatic treatment naive extremity osteosarcoma patients treated with a novel non-high dosemethotrexate-based, dose-dense combination chemotherapy regimen 'OGS-12'. Eur J Cancer 2017; 85: 49-58
- Daw NC, Neel MD, Rao BN, Billups CA, Wu J, Jenkins JJ. et al. Frontline treatment of localized osteosarcoma without methotrexate: Results of the St. Jude Children's Research Hospital OS99 trial. Cancer 2011; 117: 2770-8
- Nataraj V, Rastogi S, Khan SA, Sharma MC, Agarwala S, Vishnubhatla S. et al. Prognosticating metastatic osteosarcoma treated with uniform chemotherapy protocol without high dose methotrexate and delayed metastasectomy: A single center experience of 102 patients. Clin Transl Oncol 2016; 18: 937-44
- Shirke-Satpute SN, Rekhi B, Desai SS, Puri A, Agarwal M, Jambhekar NA. Correlation of MDM2, p53 and MIB-1 Expression with Response to Neoadjuvant Chemotherapy in High-Grade Extremity Osteosarcomas and Clinical Outcome.In: Laboratory Investigation. Nature Publishing Group 75 Varick ST, 9th Flr, New York: 10013-1917 USA; 2013.p.20A
- Bielack SS, Kempf-Bielack B, Delling G, Exner GU, Flege S, Helmke K. et al. Prognostic factors in high-grade osteosarcoma of the extremities or trunk: An analysis of 1702 patients treated on neoadjuvant cooperative osteosarcoma study group protocols. J Clin Oncol 2002; 20: 776-90
- Bacci G, Bertoni F, Longhi A, Ferrari S, Forni C, Biagini R. et al. Neoadjuvant chemotherapy for high-grade central osteosarcoma of the extremity. Histologic response to preoperative chemotherapy correlates with histologic subtype of the tumor. Cancer 2003; 97: 3068-75
- Vijayanarasimha D, Nayanar SK, Vikram S, Patil VM, Babu S, Satheesan B. Clinico-pathological Study of Limb Salvage Surgery for Osteosarcoma: Experience in a Rural Cancer Center. Indian J Surg Oncol 2017; 8: 136-41
Address for correspondence
Publication History
Received: 26 July 2018
Accepted: 02 January 2020
Article published online:
28 June 2021
© 2020. Indian Society of Medical and Paediatric Oncology. This is an open access article published by Thieme under the terms of the Creative Commons Attribution-NonDerivative-NonCommercial-License, permitting copying and reproduction so long as the original work is given appropriate credit. Contents may not be used for commercial purposes, or adapted, remixed, transformed or built upon. (https://creativecommons.org/licenses/by-nc-nd/4.0/.)
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India
- 1 Ries LA, Smith MA, Gurney JG, Linet M, Tamra T, Young JL. et al.editors. Cancer. Incidence and Survival among Children and Adolescents: United States SEER Program 1975-1995. Bethesda, MD: National Cancer Institute, SEER Program. NIH Pub.No.99-4649; 1999
- 2 Anninga JK, Gelderblom H, Fiocco M, Kroep JR, Taminiau AH, Hogendoorn PC. et al. Chemotherapeutic adjuvant treatment for osteosarcoma: Where do we stand?. Eur J Cancer 2011; 47: 2431-45
- 3 Peabody TD, Gibbs CPJr, Simon MA. Evaluation and staging of musculoskeletal neoplasms. J Bone Joint Surg Am 1998; 80: 1204-18
- 4 Enneking WF. A system for evaluation of the surgical management of musculoskeletal tumors. In: Enneking WF. editors Limb Salvage in Musculoskeletal Oncology. New York: Churchill Livingstone; 1987. p. 145
- 5 Goorin AM, Schwartzentruber DJ, Devidas M, Gebhardt MC, Ayala AG, Harris MB. et al. Presurgical chemotherapy compared with immediate surgery and adjuvant chemotherapy for nonmetastatic osteosarcoma: Pediatric Oncology Group Study POG-8651. J Clin Oncol 2003; 21: 1574-80
- 6 Bacci G, Forni C, Longhi A, Ferrari S, Mercuri M, Bertoni F. et al. Local recurrence and local control of non-metastatic osteosarcoma of the extremities: A 27-year experience in a single institution. J Surg Oncol 2007; 96: 118-23
- 7 Grimer RJ, Taminiau AM, Cannon SR. et al. Surgical Subcommitte of the European Osteosarcoma Intergroup. Surgical outcomes in osteosarcoma. J Bone Joint Surg Br 2002; 84: 395-400
- 8 Wittig JC, Bickels J, Kellar-Graney KL, Kim FH, Malawer MM. Osteosarcoma of the proximal humerus: Long-term results with limb-sparing surgery. Clin Orthop Relat Res 2002; 397: 156-76
- 9 Sukumaran RK, Rajeshwari B, Sugath S, Chellappan SG, Thankamony P, Parukuttyamma K. Methotrexate free chemotherapy and limb salvage surgery for paediatric osteosarcoma in India. Indian J Orthop 2018; 52: 58-64
- 10 Isakoff MS, Bielack SS, Meltzer P, Gorlick R. Osteosarcoma: Current treatment and a collaborative pathway to success. J Clin Oncol 2015; 33: 3029-35
- 11 Patel SJ, Lynch JWJr, Johnson T, Carroll RR, Schumacher C, Spanier S. et al Dose-intense ifosfamide/doxorubicin/cisplatin based chemotherapy for osteosarcoma in adults. Am J Clin Oncol 2002; 25: 489-95
- 12 Piperno-Neumann S, Bui B, Blay J, Roché H, Pichon F, Peny A. et al. A multicentric prospective study of intensive induction chemotherapy (API-AI) in localized osteosarcoma patients: Results of a phase II trial coordinated by the French Sarcoma Group (FSG) and the FNCLCC BECT. J Clin Oncol 2006; 24 Suppl 18: 9521
- 13 Tunn PU, Reichardt P. Chemotherapy for osteosarcoma without high-dose methotrexate: A 12-year follow-up on 53 patients. Onkologie 2007; 30: 228-32
- 14 Assi H, Missenard G, Terrier P, Le PechouxC, Bonvalot S, Vanel D. et al. Intensive induction chemotherapy without methotrexate in adult patients with localized osteosarcoma: Results of the Institut Gustave-Roussy phase II trial. Curr Oncol 2010; 17: 23-31
- 15 Bajpai J, Chandrasekharan A, Talreja V, Simha V, Chandrakanth MV, Rekhi B. et al. Outcomes in non-metastatic treatment naive extremity osteosarcoma patients treated with a novel non-high dosemethotrexate-based, dose-dense combination chemotherapy regimen 'OGS-12'. Eur J Cancer 2017; 85: 49-58
- 16 Daw NC, Neel MD, Rao BN, Billups CA, Wu J, Jenkins JJ. et al. Frontline treatment of localized osteosarcoma without methotrexate: Results of the St. Jude Children's Research Hospital OS99 trial. Cancer 2011; 117: 2770-8
- 17 Nataraj V, Rastogi S, Khan SA, Sharma MC, Agarwala S, Vishnubhatla S. et al. Prognosticating metastatic osteosarcoma treated with uniform chemotherapy protocol without high dose methotrexate and delayed metastasectomy: A single center experience of 102 patients. Clin Transl Oncol 2016; 18: 937-44
- 18 Shirke-Satpute SN, Rekhi B, Desai SS, Puri A, Agarwal M, Jambhekar NA. Correlation of MDM2, p53 and MIB-1 Expression with Response to Neoadjuvant Chemotherapy in High-Grade Extremity Osteosarcomas and Clinical Outcome.In: Laboratory Investigation. Nature Publishing Group 75 Varick ST, 9th Flr, New York: 10013-1917 USA; 2013.p.20A
- 19 Bielack SS, Kempf-Bielack B, Delling G, Exner GU, Flege S, Helmke K. et al. Prognostic factors in high-grade osteosarcoma of the extremities or trunk: An analysis of 1702 patients treated on neoadjuvant cooperative osteosarcoma study group protocols. J Clin Oncol 2002; 20: 776-90
- 20 Bacci G, Bertoni F, Longhi A, Ferrari S, Forni C, Biagini R. et al. Neoadjuvant chemotherapy for high-grade central osteosarcoma of the extremity. Histologic response to preoperative chemotherapy correlates with histologic subtype of the tumor. Cancer 2003; 97: 3068-75
- 21 Vijayanarasimha D, Nayanar SK, Vikram S, Patil VM, Babu S, Satheesan B. Clinico-pathological Study of Limb Salvage Surgery for Osteosarcoma: Experience in a Rural Cancer Center. Indian J Surg Oncol 2017; 8: 136-41


 PDF
PDF  Views
Views  Share
Share

