Primary pulmonary neoplasms in children: A report of five cases
CC BY-NC-ND 4.0 · Indian J Med Paediatr Oncol 2011; 32(04): 223-226
DOI: DOI: 10.4103/0971-5851.95146
Abstract
Primary pulmonary neoplasms are uncommon in children and represent a wide spectrum of pathology from benign to malignant. They are quite different in their histopathologic distribution from that of adults. This study was done to analyze the histopathologic spectrum of primary lung tumors in children. All the resected specimens of lung in children over a period of 5 years were studied and only the cases of primary pulmonary neoplasms were further analyzed. There were two cases of inflammatory myofibroblastic tumor. The patients were boys aged 10 and 12 years, respectively. One case of bronchial carcinoid was diagnosed in a boy of 12 years. There were one case each of pleuropulmonary blastoma (PPB) in a girl of 9 years and pulmonary blastoma (PB) in a girl of 2 years of age. In our study, the two cases of inflammatory myofibroblastic tumor had excellent prognosis. However, the cases of PPB and PB were both associated with poor clinical outcome, whereas the case of bronchial carcinoid has been doing well on follow-up.
Publication History
Article published online:
06 August 2021
© 2011. Indian Society of Medical and Paediatric Oncology. This is an open access article published by Thieme under the terms of the Creative Commons Attribution-NonDerivative-NonCommercial-License, permitting copying and reproduction so long as the original work is given appropriate credit. Contents may not be used for commercial purposes, or adapted, remixed, transformed or built upon. (https://creativecommons.org/licenses/by-nc-nd/4.0/.)
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India
Abstract
Primary pulmonary neoplasms are uncommon in children and represent a wide spectrum of pathology from benign to malignant. They are quite different in their histopathologic distribution from that of adults. This study was done to analyze the histopathologic spectrum of primary lung tumors in children. All the resected specimens of lung in children over a period of 5 years were studied and only the cases of primary pulmonary neoplasms were further analyzed. There were two cases of inflammatory myofibroblastic tumor. The patients were boys aged 10 and 12 years, respectively. One case of bronchial carcinoid was diagnosed in a boy of 12 years. There were one case each of pleuropulmonary blastoma (PPB) in a girl of 9 years and pulmonary blastoma (PB) in a girl of 2 years of age. In our study, the two cases of inflammatory myofibroblastic tumor had excellent prognosis. However, the cases of PPB and PB were both associated with poor clinical outcome, whereas the case of bronchial carcinoid has been doing well on follow-up.
INTRODUCTION
Primary pulmonary neoplasms are not common in children. Amongst the malignant pulmonary lesions in children, secondaries from osteogenic sarcomas, Wilms’ tumors, and rhabdomyosarcomas are more commonly encountered.[1] The pediatric primary pulmonary neoplasms represent a wide range of pathology from benign to malignant end of the spectrum. They are quite different in their histopathologic distribution from that of adults. Amongst the variety of benign and malignant tumors arising in adults, majority are carcinomas; squamous cell carcinomas and adenocarcinomas constitute the bulk.[2] However, in children, inflammatory myofibroblastic tumors, chondromatous hamartomas, granular cell myoblastomas, leiomyomas, bronchial chondromas and teratomas constitute the major bulk of benign tumors, whereas carcinoids, mucoepidermoid carcinomas, pleuropulmonary blastomas (PPBs), pulmonary blastomas (PBs), adenoid cystic carcinomas, sarcomas like fibrosarcomas, leiomyosarcomas, rhabdomyosarcomas constitute the list of malignant lung tumors. As data of primary pulmonary neoplasms are limited in different pediatric centers of our country, this report highlights the prevalence of these tumors in our setting.
We came across five cases of these rare primary pulmonary neoplasms in children in last 5 years, which have been presented in this report.
This was a retrospective analysis of data from two pediatric centers, collected over last 5 years (2004–2009). Details of all cases of lung resection and lobectomy were noted and only those cases of primary non-hematologic lung tumors were incorporated. There were five cases of primary lung tumors and two cases of metastatic Wilm's tumor.
CASE REPORTS
Five children (three boys and two girls) were diagnosed with primary pulmonary neoplasms. The average age at presentation was 9.2 years, with the age ranging from 2 to 12 years. Presenting complaints included cough and fever (five cases), recurrent chest infections (three cases), hemoptysis (one case) and one case presented masquerading as empyema.
Cases 1 and 2
A boy, 10 years of age, came with fever and unresolving chest infection. He underwent left lower lobectomy for suggested tumor on computed tomography (CT) scan. On gross examination, a 6-cm gray-white, firm, well-demarcated mass was noted [Figure 1a]. Microscopically, the mass was entirely composed of spindle-shaped fibroblastic cells arranged loosely with dense lymphocytic and plasmacytic infiltration, some of which contained Russel bodies [Figure 1b]. Areas of hemorrhage, fibrosis, and hyalinization were present and a diagnosis of inflammatory myofibroblastic tumor was made. Patient is doing well on follow-up. A second case was that of a 12-year-old boy who also underwent a lobectomy for similar clinical and radiological features. The histology of the tumor revealed it to be an inflammatory myofibroblastic tumor.
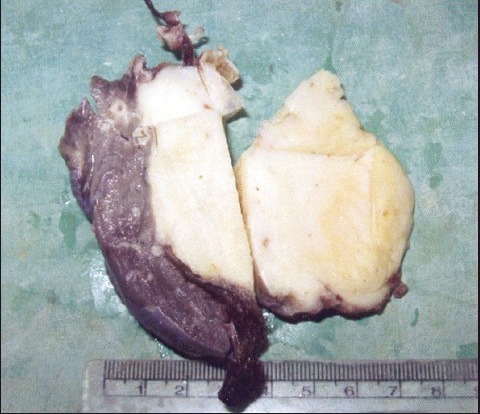
| Figure 1a:Gross photograph of the excised right lower lobe of the lung containing the tumor, inflammatory myofibroblastic tumor

| Figure 1b:Photomicrograph of the inflammatory myofibroblastic tumor showing proliferating fibroblasts and collagen bundles infiltrated by inflammatory cells like plasma cells and lymphocytes (H and E, ×100)
Case 3
A boy aged 12 years presented with recurrent hemoptysis and underwent right-sided pneumonectomy for a lung mass seen on CT scan. Gross examination of the lung showed a well-circumscribed, 3.5-cm nodular mass in the bronchial wall with ulceration. Elsewhere, the lungs showed features of collapse. Microscopic examination of the tumor showed nests and trabeculae of uniform, small cells separated by delicate fibrovascular septae. The cells had round nuclei with finely granular chromatin and inconspicuous nucleoli. There was no evidence of necrosis, mitosis or atypia [Figure 1c]. A diagnosis of bronchial carcinoid was made. The boy is doing well on follow-up.
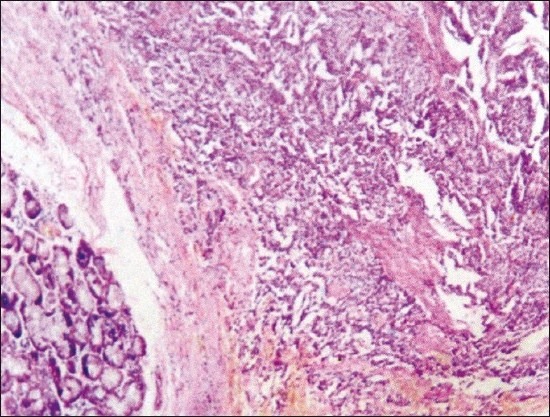
| Figure 1c:Photomicrograph of the case of bronchial carcinoid showing bronchial glands in the lower left corner. The tumor is composed of nests and trabeculae of uniform small cells separated by delicate fibrous septae (H and E, ×100)
Case 4
A 2-year-old girl presented with massive hemoptysis. She had received oral medications without improvement in her symptoms. Radiological investigations which included CT scan revealed a mass invading the lower left lung. Left lower lobectomy was done. Fragments of gray-white, soft mass were received. Histopathologic examination showed immature, pleomorphic, small, dark blastemal cells forming tubules in a loose fibrous stromal background. The cells showed brisk mitosis. There was evidence of bone and cartilage formation [Figure 2a]. No pleural involvement was found. A diagnosis of PB was made. She recovered after the operation and was given four cycles of chemotherapy with PLADO regimen. She had severe hemoptysis and died 1 year after the surgery.
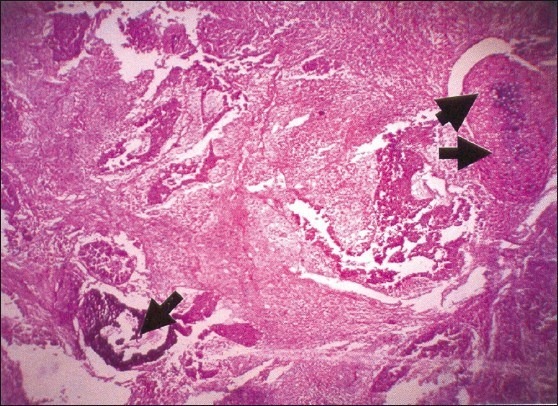
| Figure 2a:Photomicrograph of pulmonary blastoma showing a tumor composed of small, darkly staining blastemal cells. There is also evidence of bone (single arrow head) and cartilage formation (double arrow heads) (H and E, ×100)
Case 5
A girl of 9 years came with empyema not responding to medical treatment. At thoracotomy, a thick pleural mass encasing the lungs with cystic change was found and it was decorticated in a piecemeal manner. Microscopic examination showed that the mass involved only pleura and was composed of sheets of small, darkly staining, round to oval shaped primitive cells with brisk mitotic activity. Cells were arranged in highly cellular areas alternating with looser myxoid areas with no epithelial component or any other line of differentiation. Immunohistochemistry was done for vimentin and CD 99 (MIC2). The cells were uniformly vimentin positive and negative for MIC2 [Figure 2b]. A diagnosis of PPB was made.
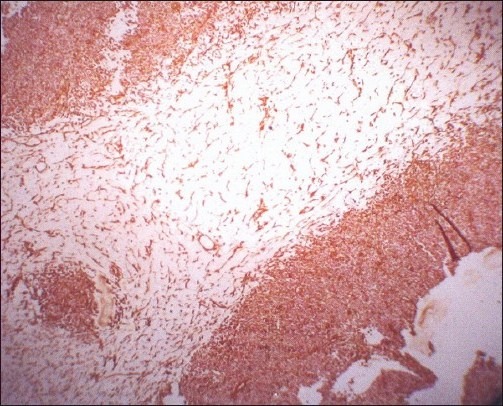
| Figure 2b:Immunoperoxidase stain showing strong cytoplasmic positivity for vimentin (original, ×100)
Following operation, she was given few cycles of chemotherapy. However, she succumbed 4 months later.
DISCUSSION
Primary pulmonary tumors of childhood are rare and histopathologically diverse.[3] It is important to make a correct diagnosis as not all of them are malignant in nature. Lack of familiarity with these entities can cause errors in diagnosis. Inflammatory myofibroblastic tumors and carcinoids are associated with excellent prognosis, whereas PPBs continue to have dismal prognosis despite tremendous advances in newer chemotherapeutic regimes.
Inflammatory myofibroblastic tumor has a number of pseudonyms. Some of them are inflammatory pseudotumor, plasma cell granuloma, fibroxanthoma, histiocytoma, sclerosing hemangioma, hyalinizing granuloma, etc. Though a rare tumor, it is the most common benign tumor of the lung in the pediatric age group, accounting for 84% of the cases. It is predominantly seen in older children, without any sex predilection.[4,5] The etiology of these tumors is not certain and often difficult to ascertain. The current understanding of pathophysiology is based on an abnormal response of myofibroblasts to tissue injury. Associated pulmonary infection has been reported in 30%-cases. Chest X-ray typically demonstrates a solitary, well-demarcated peripheral lung mass of average size ranging from 3 to 10 cm.[4] Rarely, they present as polypoid endobronchial mass. Histologically, the tumor is composed of proliferating fibroblasts, collagen infiltrated with various inflammatory cells like plasma cells, lymphocytes, histiocytes, etc. There is fibrosis and intra-alveolar fibrosis, especially at the margin of the lesion. The tumor mainly needs to be differentiated from lymphoma, plasmacytoma, organizing pneumonia and the rare malignant fibrous histiocytoma. Complete resection, wherever possible, is the standard therapy and results in excellent survival.[6] Long-term follow-up is recommended, especially in cases of incomplete resection. Recurrence following incomplete resection has been reported. Of late, nonsteroidal anti-inflammatory drugs are being put to good use in large lesions.
Pulmonary carcinoids, previously known as bronchial adenoma, constitute a major portion of malignant lung tumors in children. Presenting symptoms are cough, pneumonitis and hemoptysis as they are centrally located. Misinterpretation of symptoms may result in delay in diagnosis. Radiological evaluation along with bronchoscopy aid in the diagnosis. Carcinoids fall under the category of neuroendocrine tumors along with small cell carcinoma, atypical carcinoid, and large cell neuroendocrine carcinoma. Lack of mitosis, atypia, and necrosis help to distinguish them from atypical carcinoids. Treatment of choice is operative with the aim to conserve as much functioning lung tissue as possible.[7] Because of relatively low malignant potential of carcinoid tumors, results of surgical treatment are excellent even in the presence of metastasis to the regional lymph nodes.[8]
Unlike the other three common blastomas (i.e. hepatoblastoma, nephroblastoma, retinoblastoma) which occur in children, PB is mainly adult tumor. Originally termed “embryoma” by Bernard in 1952, the term blastoma was introduced in 1961 by Spencer. It is a rare, aggressive primary malignant lung neoplasm, originating from multipotential pulmonary cells composed of immature mesenchyme and epithelium which is morphologically similar to fetal lung tissue. It represents 0.25–0.5% of all primary lung tumors. 20–25% of these tumors present in childhood.[9] It has a male preponderance, but our case was a girl. Right and left lungs are equally affected. The usual clinical presentation of PB is fever, dyspnea, cough, hemoptysis and even non-resolving pneumonia. Lack of acquaintance of this entity often leads to treatment for other causes. Radiological finding includes a cystic or solid lung mass with variable contrast enhancement. Lobectomy along with lymph node removal, where applicable, is the treatment of choice. Also, the tumor shows a potential for radio-chemosensitivity.[10] Microscopically, blastematous, epithelial and stromal elements are present in varying proportion. The blastematous element is composed of small cells with dark, oval nuclei, and scant cytoplasm arranged around blood vessels. Epithelial differentiation, which can be heterogeneous in appearance, includes cribriform pattern, morules, tubular and glandular patterns. Stromal components include bone and cartilage. Pleomorphism, necrosis, and atypical mitosis are frequently seen. These aggressive tumors tend to relapse locally and metastasize to distant sites and also to lymph nodes. The 5-year survival rate is about 16%.[11] Prognosis is worse if the tumor is more than 5 cm at presentation.[12]
PPB is a rare and aggressive intrathoracic malignant tumor occurring in 20% of cases less than 20 years of age and 75% of these occur under 10 years of age. There is a distinct male predominance at all ages. Majority of the patients present with nonproductive cough and fever. Cases with predominant pleural involvement often present with pleuritis and empyema refractory to medical treatment like our case 5. They may arise from lung, pleura or mediastinum even in extralobar sequestration of lung, mostly in right hemithorax as in our case. Radiological imaging demonstrates a solid to cystic lesion with hemorrhage and necrosis. PPB, as opposed to adult PB, lacks an epithelial component entirely. The mesenchymal component may show heterologous differentiation like rhabdomyosarcoma, chondrosarcoma, etc. However, our case had only sheets of primitive cell component. Negative CD99, as in ours, helps to distinguish this from other important small round cell tumors of childhood like primitive neuroectodermal tumors. Resection is warranted in all cases. Recurrences and metastases are commonly seen. Prognosis for long-term survival is 25–50%.[13] Nowadays, resection followed by multimodal neoadjuvant chemotherapy and radiotherapy is the treatment of choice in more extensive disease with dissemination.
CONCLUSION
Primary pulmonary lung tumors are rare in children and comprise a unique spectrum of lesions often distinct from adult population.[14] In this series, it was found that inflammatory pseudotumors are the most commonly encountered tumor of this age group.
ACKNOWLEDGMENT
We are grateful to Prof. Subir K Chatterjee, Park Children's Center for Treatment and Research, for giving us the details of case 4.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- Cohen MC, Kaschula RO. Primary pulmonary tumors in childhood: A review of 31 years′ experience and the literature. Pediatr Pulmonol 1992;14:222-32.
- Husain NA, Kumar V. The Lung. In: Kumar V, Abbas KA, Fausto N (editors). Pathologic Basis Of Diseases. Pennsylvania: Elsevier; 2004. p. 759.
- Yu DC, Grabowski MJ, Kozakewich HP, Perez-Atayde AR, Voss SD, Shamberger RC, et al. Primary lung tumors in children and adolescents: A 90-year experience. J Pediatr Surg 2010;45:1090-5.
- Monzon CM, Gilchrist GS, Burgert EO Jr, O′Connell EJ, Telander RL, Hoffman AD, et al. Plasma cell granuloma of the lung in children. Pediatrics 1982;70:268-74.
- Berardi RS, Lee SS, Chen HP, Stines GJ. Inflammatory pseudotumors of the lung. Surg Gynecol Obstet 1983;156:89-96.
- Cerfolio RJ, Allen MS, Nascimento AG, Deschamps C, Trastek VF, Miller DL, et al. Inflammatory pseudotumors of the lung. Ann Thorac Surg 1999;67:933-6.
- Keller KM, Helwig H. Carcinoid tumor of the lung in childhood-review and case report. Klin Padiatr 1981;193:355-61.
- Martini N, Zaman MB, Bains MS, Burt ME, McCormack PM, Rusch VW, et al. Treatment and prognosis in bronchial carcinoids involving regional lymph nodes. J Thorac Cardiovasc Surg 1994;107:1-7.
- Karnak I, Ciftci AO, Senocak ME, Kale G, Buyukpamukcu N. Pulmonary Blastoma: Diagnostic and therapeutic aspects. Pediatr Surg Int 1999;15:380-2.
- Buchel H, Casella R, Muller W, Martinelli G, Cavalli F, Luscieti P. Blastoma of the lung-a rare malignancy. Case report. Helv Chir Acta 1993;60:21-5.
- Thomas SJ. The respiratory Tract. In: Thomas JS, Dehner LP (editors). Pediatric pathology. Philadelphia: JB Lippincott Co; 1992. p. 561.
- Walker RI, Suvarna K, Matthews S. Pulmonary blastoma: Presentation of two atypical cases and review of literature. Br J Radiol 2005;78:437-40.
- Manivel JC, Priest JR, Watterson J, Steiner M, Woods WG, Wick MR, et al. Pleuropulmonary blastoma: The so called pulmonary blastoma of childhood. Cancer 1988;62:1516-26.
- Dishop MK, Kuruvilla S. Primary and metastatic lung tumors in the pediatric population: A review and 25-year experience at a large children′s hospital. Arch Pathol Lab Med 2008;132:1079-103.

| Figure 1a:Gross photograph of the excised right lower lobe of the lung containing the tumor, inflammatory myofibroblastic tumor

| Figure 1b:Photomicrograph of the inflammatory myofibroblastic tumor showing proliferating fibroblasts and collagen bundles infiltrated by inflammatory cells like plasma cells and lymphocytes (H and E, ×100)

| Figure 1c:Photomicrograph of the case of bronchial carcinoid showing bronchial glands in the lower left corner. The tumor is composed of nests and trabeculae of uniform small cells separated by delicate fibrous septae (H and E, ×100)

| Figure 2a:Photomicrograph of pulmonary blastoma showing a tumor composed of small, darkly staining blastemal cells. There is also evidence of bone (single arrow head) and cartilage formation (double arrow heads) (H and E, ×100)

| Figure 2b:Immunoperoxidase stain showing strong cytoplasmic positivity for vimentin (original, ×100)
References
- Cohen MC, Kaschula RO. Primary pulmonary tumors in childhood: A review of 31 years′ experience and the literature. Pediatr Pulmonol 1992;14:222-32.
- Husain NA, Kumar V. The Lung. In: Kumar V, Abbas KA, Fausto N (editors). Pathologic Basis Of Diseases. Pennsylvania: Elsevier; 2004. p. 759.
- Yu DC, Grabowski MJ, Kozakewich HP, Perez-Atayde AR, Voss SD, Shamberger RC, et al. Primary lung tumors in children and adolescents: A 90-year experience. J Pediatr Surg 2010;45:1090-5.
- Monzon CM, Gilchrist GS, Burgert EO Jr, O′Connell EJ, Telander RL, Hoffman AD, et al. Plasma cell granuloma of the lung in children. Pediatrics 1982;70:268-74.
- Berardi RS, Lee SS, Chen HP, Stines GJ. Inflammatory pseudotumors of the lung. Surg Gynecol Obstet 1983;156:89-96.
- Cerfolio RJ, Allen MS, Nascimento AG, Deschamps C, Trastek VF, Miller DL, et al. Inflammatory pseudotumors of the lung. Ann Thorac Surg 1999;67:933-6.
- Keller KM, Helwig H. Carcinoid tumor of the lung in childhood-review and case report. Klin Padiatr 1981;193:355-61.
- Martini N, Zaman MB, Bains MS, Burt ME, McCormack PM, Rusch VW, et al. Treatment and prognosis in bronchial carcinoids involving regional lymph nodes. J Thorac Cardiovasc Surg 1994;107:1-7.
- Karnak I, Ciftci AO, Senocak ME, Kale G, Buyukpamukcu N. Pulmonary Blastoma: Diagnostic and therapeutic aspects. Pediatr Surg Int 1999;15:380-2.
- Buchel H, Casella R, Muller W, Martinelli G, Cavalli F, Luscieti P. Blastoma of the lung-a rare malignancy. Case report. Helv Chir Acta 1993;60:21-5.
- Thomas SJ. The respiratory Tract. In: Thomas JS, Dehner LP (editors). Pediatric pathology. Philadelphia: JB Lippincott Co; 1992. p. 561.
- Walker RI, Suvarna K, Matthews S. Pulmonary blastoma: Presentation of two atypical cases and review of literature. Br J Radiol 2005;78:437-40.
- Manivel JC, Priest JR, Watterson J, Steiner M, Woods WG, Wick MR, et al. Pleuropulmonary blastoma: The so called pulmonary blastoma of childhood. Cancer 1988;62:1516-26.
- Dishop MK, Kuruvilla S. Primary and metastatic lung tumors in the pediatric population: A review and 25-year experience at a large children′s hospital. Arch Pathol Lab Med 2008;132:1079-103.


 PDF
PDF  Views
Views  Share
Share

