Primary Renal Cell Lymphoma: Case Report, Diagnosis, and Management
CC BY-NC-ND 4.0 · Indian J Med Paediatr Oncol 2017; 38(04): 545-547
DOI: DOI: 10.4103/ijmpo.ijmpo_167_16
Abstract
The symptoms of primary renal lymphoma (PRL) may mimic a renal cell carcinoma. Since the diagnosis is mostly after a radical nephrectomy, we recommend a percutaneous biopsy or cytology from the renal mass in patients who have features suggestive of a lymphoma. A magnetic resonance imaging may give an image more specific for a lymphoma. There are no clinical trials for the treatment of PRL, but all previously published case reports used R-CHOP and a few patients did better than the median survival of 6 months.
Keywords
Non-Hodgkin lymphoma - positron emission tomography-magnetic resonance imaging - renal lymphomaPublication History
Article published online:
04 July 2021
© 2017. Indian Society of Medical and Paediatric Oncology. This is an open access article published by Thieme under the terms of the Creative Commons Attribution-NonDerivative-NonCommercial-License, permitting copying and reproduction so long as the original work is given appropriate credit. Contents may not be used forcommercial purposes, or adapted, remixed, transformed or built upon. (https://creativecommons.org/licenses/by-nc-nd/4.0/.)
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India
Abstract
The symptoms of primary renal lymphoma (PRL) may mimic a renal cell carcinoma. Since the diagnosis is mostly after a radical nephrectomy, we recommend a percutaneous biopsy or cytology from the renal mass in patients who have features suggestive of a lymphoma. A magnetic resonance imaging may give an image more specific for a lymphoma. There are no clinical trials for the treatment of PRL, but all previously published case reports used R-CHOP and a few patients did better than the median survival of 6 months.
Introduction
Diffuse large B-cell lymphoma (DLBCL) is the most common subtype of non–Hodgkin's lymphoma (NHL), and constitutes 25% of all NHL cases.[1] The disease usually presents with a rapidly enlarging symptomatic mass,[2] and the diagnosis is made on a excision biopsy. It is a morphological and immunophenotypical diagnosis.
Renal NHL is most commonly a part of a disseminated disease, and primary renal lymphoma (PRL) is thought to be a rare disease. There are many case reports on PRL, and DLBCL is the most common type of PRL.[3] Clinically and radiologically, PRL may be confused with renal cell carcinoma (RCC).[4]
We present a patient who came with a diagnosis of RCC, but on workup, the diagnosis was changed to PRL after a fine-needle aspiration cytology (FNAC).
Case Report
A 64-year-old female patient presented to the clinical with a history of pain abdomen, localized in the flank, and loss of appetite for 1 month. She had documented a loss of weight of 3 kg. On examination, a right renal lump was palpable. Computed tomography (CT) scan of the abdomen was done before presentation, which showed a large 8.9 cm × 8.6 cm mixed density mass in the right kidney. The patient presented to us with a diagnosis of RCC, for further evaluation and management. To confirm the diagnosis, we did ultrasound guided FNAC from the right renal mass that showed large round cleaved nuclei, coarse chromatin, and scanty dense blue cytoplasm. This was suggestive of NHL. Positron emission tomography–magnetic resonance imaging (PET-MRI) was done to see evidence of metastasis, which showed fluorodeoxyglucose avid thyroid lesion, right renal mass [Figure 1] with metabolically active subcentimeter nodes in the cervical, mediastinal, and enlarged discrete and confluent retroperitoneal and mesenteric nodes. An excision biopsy from a cervical node was done to classify the disease. Histopathology confirmed DLBCL, which was CD20 positive and Mib-1 labeling index of 80% [Figure 2]. The patient was started on chemotherapy with R-CHOP, which she tolerated well for three cycles. Repeat PET scan was done which showed regression of primary renal lesion and absence of any other lymph node.
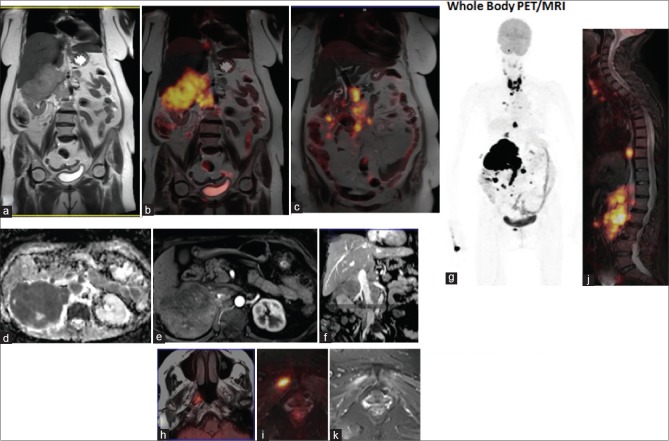
| Figure 1:Whole body positron emission tomography-magnetic resonance imaging of the patient. Mass lesion is seen replacing the upper mid pole of right kidney with intence FDG uptake (SUV Max 26.4) (T2W coronal MR: a & Coronal PET/MRI: b) with marked diffusion restriction (DWI MR: d) missing any striking arterial phase blush (arterial phase CE MR: e). Note: Enlarged confluent right renal hilar, mesentric and retroperitoneal nodes with high FDG uptake (SUV Max ∼32) and diffusion restriction (b, d & PET/MRI: c) encasing right renal pedicle effacing flow in right renal vein & renal IV with no thrombus (CE MRA: f). Multiple avid mediastinal nodes, active right lung lesion & the thyrod (g: WB MIP PET) and soft tissue lesion in right nasopharynx (PET/MRI: h). No osseous lesion seen (sagittal PET/MRI spine: j). The avid lesion in the pelvis (PET/MRI: k) is a soft tissue deposit in the right obturator externus muscle (STIR MRI: i)

| Figure 2:Histology of the tumor specimen (a) H and E, (b) CD20 immunohistochemistry, (c) MiB labeling
Discussion
This is a case report describing a case of primary NHL of the kidney. A renal lump was found in the examination, and after imaging using CT scan, the patient was planned for a nephrectomy with a probable diagnosis of RCC. The patient had symptoms of abdominal pain and a palpable mass in the abdomen, which was also suggestive of RCC. The contrast-enhanced CT scan showed a lump with a large lesion of mixed density, which could be present in an RCC. We did a cytopathology test due to previous experience at our center where the biopsy from the nephrectomy specimen showed renal lymphoma. Cytopathology of this patient confirmed our suspicion of PRL.
Renal involvement of NHL is usually part a disseminated disease, and PRL is NHL from renal parenchyma and not invasion from adjacent lymphomatous mass.[3] The disease usually affects adults over the age of 60 years of age. Severe histogenetic theories have been postulated since there is no lymphoid tissue present in the kidney, these are: a possible origin in subscapular lymphatics, possible hematogenous seeding in the kidney, or an extension of retroperitoneal disease.[4]
PRL is often confused for RCC, which means that the diagnosis is usually made after radical nephrectomy.[5] The clinical presentation may be similar to the other renal malignancies. Flank or abdominal pain is the most frequent symptom.[6] However, PRL can present with proteinuria or nephrotic syndrome and rapidly progress to renal failure, especially when both kidneys are affected.[7]
Imaging may give hints about the diagnosis. Indirect signs of PRL include renal size enlargement, sinus or hilum direct infiltration by a bulky mass, or diffuse retroperitoneal infiltration. Indirect signs that may indicate an RCC include the following: the presence of calcifications, venous thrombosis, or an obstructive mass effect over the renal vessels or urinary tract.[6] These signs are indirect, and it is always required to confirm the diagnosis with a percutaneous biopsy. MRI and CT are considered to have the same accuracy for detection and characterization of the most renal lesion.[8,9] MRI is the modality of choice in those who will not be able to take iodinated contrast.
There are no clinical trials on the treatment of PRL, but all the case reports we reviewed treated the patient with CHOP and rituximab.[3,6,7,10,11] The mean survival of the patient is known to be 6 months only.[10]
The symptoms of PRL may mimic an RCC. Since the diagnosis is mostly after a radical nephrectomy, we recommend a percutaneous biopsy or cytology from the renal mass in patients who have features suggestive of a lymphoma. The features suggestive of lymphoma include common symptoms such as proteinuria or lymphadenopathy. We believe that an MRI gave an image more specific for a lymphoma. The MRI we did as part of the PET-MRI gave a more characteristic picture of lymphoma which the CT scan could not. A PET scan helped us pick up sub-centimeter metabolically active nodules, which helped us with the diagnosis. We excised a superficial lymph node in the cervical area to confirm the diagnosis. We did not find any clinical trials for the treatment, but all previously published case reports used R-CHOP, and a few patients did better than the median survival of 6 months.
Conclusion
Thus, we conclude that a tissue diagnosis and an MRI should be done in patients with a renal lump to rule out other diagnosis.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Morton LM, Wang SS, Devesa SS, Hartge P, Weisenburger DD, Linet MS, et al. Lymphoma incidence patterns by WHO subtype in the United States, 1992-2001. Blood 2006;107:265-76.
- Hui D, Proctor B, Donaldson J, Shenkier T, Hoskins P, Klasa R, et al. Prognostic implications of extranodal involvement in patients with diffuse large B-cell lymphoma treated with rituximab and cyclophosphamide, doxorubicin, vincristine, and prednisone. Leuk Lymphoma 2010;51:1658-67.
- Okuno SH, Hoyer JD, Ristow K, Witzig TE. Primary renal non-Hodgkin's lymphoma. An unusual extranodal site. Cancer 1995;75:2258-61.
- Omer HA, Hussein MR. Primary renal lymphoma. Nephrology (Carlton) 2007;12:314-5.
- Vázquez Alonso F, Sánchez Ramos C, Vicente Prados FJ, Pascual Geler M, Ruiz Carazo E, Becerra Massare P, et al. Primary renal lymphoma: Report of three new cases and literature review. Arch Esp Urol 2009;62:461-5.
- Vázquez-Alonso F, Puche-Sanz I, Sánchez-Ramos C, Flores-Martín J, Vicente-Prados J, Cózar-Olmo JM, et al. Primary renal lymphoma: Long-term results of two patients treated with a chemotherapy + rituximab protocol. Case Rep Oncol Med 2012;2012:726424.
- Stallone G, Infante B, Manno C, Campobasso N, Pannarale G, Schena FP, et al. Primary renal lymphoma does exist: Case report and review of the literature. J Nephrol 2000;13:367-72.
- Sheth S, Ali S, Fishman E. Imaging of renal lymphoma: Patterns of disease with pathologic correlation. Radiographics 2006;26:1151-68.
- Nikken JJ, Krestin GP. MRI of the kidney-state of the art. Eur Radiol 2007;17:2780-93.
- Geetha N, Shahid A, Rajan V, Jacob PM. Primary renal lymphoma – A case report. Ecancermedicalscience 2014;8:466.
- Porcaro AB, D'Amico A, Novella G, Curti P, Ficarra V, Antoniolli SZ, et al. Primary lymphoma of the kidney. Report of a case and update of the literature. Arch Ital Urol Androl 2002;74:44-7.

| Figure 1:Whole body positron emission tomography-magnetic resonance imaging of the patient. Mass lesion is seen replacing the upper mid pole of right kidney with intence FDG uptake (SUV Max 26.4) (T2W coronal MR: a & Coronal PET/MRI: b) with marked diffusion restriction (DWI MR: d) missing any striking arterial phase blush (arterial phase CE MR: e). Note: Enlarged confluent right renal hilar, mesentric and retroperitoneal nodes with high FDG uptake (SUV Max ∼32) and diffusion restriction (b, d & PET/MRI: c) encasing right renal pedicle effacing flow in right renal vein & renal IV with no thrombus (CE MRA: f). Multiple avid mediastinal nodes, active right lung lesion & the thyrod (g: WB MIP PET) and soft tissue lesion in right nasopharynx (PET/MRI: h). No osseous lesion seen (sagittal PET/MRI spine: j). The avid lesion in the pelvis (PET/MRI: k) is a soft tissue deposit in the right obturator externus muscle (STIR MRI: i)

| Figure 2:Histology of the tumor specimen (a) H and E, (b) CD20 immunohistochemistry, (c) MiB labeling
References
- Morton LM, Wang SS, Devesa SS, Hartge P, Weisenburger DD, Linet MS, et al. Lymphoma incidence patterns by WHO subtype in the United States, 1992-2001. Blood 2006;107:265-76.
- Hui D, Proctor B, Donaldson J, Shenkier T, Hoskins P, Klasa R, et al. Prognostic implications of extranodal involvement in patients with diffuse large B-cell lymphoma treated with rituximab and cyclophosphamide, doxorubicin, vincristine, and prednisone. Leuk Lymphoma 2010;51:1658-67.
- Okuno SH, Hoyer JD, Ristow K, Witzig TE. Primary renal non-Hodgkin's lymphoma. An unusual extranodal site. Cancer 1995;75:2258-61.
- Omer HA, Hussein MR. Primary renal lymphoma. Nephrology (Carlton) 2007;12:314-5.
- Vázquez Alonso F, Sánchez Ramos C, Vicente Prados FJ, Pascual Geler M, Ruiz Carazo E, Becerra Massare P, et al. Primary renal lymphoma: Report of three new cases and literature review. Arch Esp Urol 2009;62:461-5.
- Vázquez-Alonso F, Puche-Sanz I, Sánchez-Ramos C, Flores-Martín J, Vicente-Prados J, Cózar-Olmo JM, et al. Primary renal lymphoma: Long-term results of two patients treated with a chemotherapy + rituximab protocol. Case Rep Oncol Med 2012;2012:726424.
- Stallone G, Infante B, Manno C, Campobasso N, Pannarale G, Schena FP, et al. Primary renal lymphoma does exist: Case report and review of the literature. J Nephrol 2000;13:367-72.
- Sheth S, Ali S, Fishman E. Imaging of renal lymphoma: Patterns of disease with pathologic correlation. Radiographics 2006;26:1151-68.
- Nikken JJ, Krestin GP. MRI of the kidney-state of the art. Eur Radiol 2007;17:2780-93.
- Geetha N, Shahid A, Rajan V, Jacob PM. Primary renal lymphoma – A case report. Ecancermedicalscience 2014;8:466.
- Porcaro AB, D'Amico A, Novella G, Curti P, Ficarra V, Antoniolli SZ, et al. Primary lymphoma of the kidney. Report of a case and update of the literature. Arch Ital Urol Androl 2002;74:44-7.


 PDF
PDF  Views
Views  Share
Share

