Prospective Observational Study of Evaluating Cisplatin-Induced Ototoxicity in Patients
CC BY-NC-ND 4.0 · Indian J Med Paediatr Oncol 2022; 43(05): 424-430
DOI: DOI: 10.1055/s-0042-1755546
Abstract
Introduction Platinum-based chemotherapeutic agents cisplatin and carboplatin are two of the most widely used drugs in cancer today. They display wide range of adverse reactions; among them, ototoxicity is an important cumulative toxicity that more commonly observed with cisplatin. At a later stage, it can affect speech of individual and lead to communication problem with decreased cognitive function and depression in cancer survivors. Periodic monitoring of hearing loss with pure-tone audiometry (PTA) provides early evidence of ototoxicity which may decrease debilitating effect of the same in a patient.
Objective The primary objective of this study was to assess cisplatin-induced ototoxicity. We also investigated its severity, reversibility, and other modifying risk factors.
Materials and Methods We conducted a prospective observational descriptive type of epidemiological study. The study was conducted over 80 randomly selected cancer patients (for estimation of sample size, the following formula was used n = [Zα 2 PQ] / d 2), who were starting with their first cycle of cisplatin from August 2018 to July 2020. This study was conducted at tertiary cancer care center in western Gujarat which caters patients from all over India. We performed PTA in all randomized patients at baseline and periodically. We classified hearing loss according to the World Health Organization (WHO) criteria.
Results A total of 30% (n = 24) patients developed cisplatin-induced ototoxicity according to WHO criteria at end of 3 months after starting the first cycle of cisplatin. It was sensory neuronal, affecting both the ears equally, and was seen predominantly at high frequency. We observed hearing loss at 3 months to be significantly more common in the 301 to 400 mg/m2 cumulative dose group (47%), as compared with the other two groups (0–200 mg/m2 and 201–300 mg/m2; p < 0.05). It showed dose dependency with cisplatin. In the multivariate step-wise regression model, baseline hearing loss (odds ratio [OR] = 17.71, 95% confidence interval [CI]: 6.57–118.91, p < 0.05) and cumulative cisplatin dose of more than 300 mg/m2 were significantly associated with hearing loss at 3 months (OR = 6.62, 95% CI: 2.33–18.74, p < 0.05).
Conclusion Cisplatin-induced ototoxicity manifests as a bilateral high frequency sensorineural hearing loss. Cumulative dose of cisplatin is an important predictor of development of ototoxicity. Baseline and periodic audiometric monitoring could detect ototoxicity early which leads to possible limitation on the severity of ototoxicity.
Keywords
cisplatin ototoxicity - ototoxicity monitoring - dose dependent toxicity - ototoxicity - pure-tone audiometry - hearing lossPublication History
Article published online:
20 October 2022
© 2022. Indian Society of Medical and Paediatric Oncology. This is an open access article published by Thieme under the terms of the Creative Commons Attribution-NonDerivative-NonCommercial License, permitting copying and reproduction so long as the original work is given appropriate credit. Contents may not be used for commercial purposes, or adapted, remixed, transformed or built upon. (https://creativecommons.org/licenses/by-nc-nd/4.0/)
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India
Abstract
Introduction Platinum-based chemotherapeutic agents cisplatin and carboplatin are two of the most widely used drugs in cancer today. They display wide range of adverse reactions; among them, ototoxicity is an important cumulative toxicity that more commonly observed with cisplatin. At a later stage, it can affect speech of individual and lead to communication problem with decreased cognitive function and depression in cancer survivors. Periodic monitoring of hearing loss with pure-tone audiometry (PTA) provides early evidence of ototoxicity which may decrease debilitating effect of the same in a patient.
Objective The primary objective of this study was to assess cisplatin-induced ototoxicity. We also investigated its severity, reversibility, and other modifying risk factors.
Materials and Methods We conducted a prospective observational descriptive type of epidemiological study. The study was conducted over 80 randomly selected cancer patients (for estimation of sample size, the following formula was used n = [Zα 2 PQ] / d 2), who were starting with their first cycle of cisplatin from August 2018 to July 2020. This study was conducted at tertiary cancer care center in western Gujarat which caters patients from all over India. We performed PTA in all randomized patients at baseline and periodically. We classified hearing loss according to the World Health Organization (WHO) criteria.
Results A total of 30% (n = 24) patients developed cisplatin-induced ototoxicity according to WHO criteria at end of 3 months after starting the first cycle of cisplatin. It was sensory neuronal, affecting both the ears equally, and was seen predominantly at high frequency. We observed hearing loss at 3 months to be significantly more common in the 301 to 400 mg/m2 cumulative dose group (47%), as compared with the other two groups (0–200 mg/m2 and 201–300 mg/m2; p < 0.05). It showed dose dependency with cisplatin. In the multivariate step-wise regression model, baseline hearing loss (odds ratio [OR] = 17.71, 95% confidence interval [CI]: 6.57–118.91, p < 0.05) and cumulative cisplatin dose of more than 300 mg/m2 were significantly associated with hearing loss at 3 months (OR = 6.62, 95% CI: 2.33–18.74, p < 0.05).
Conclusion Cisplatin-induced ototoxicity manifests as a bilateral high frequency sensorineural hearing loss. Cumulative dose of cisplatin is an important predictor of development of ototoxicity. Baseline and periodic audiometric monitoring could detect ototoxicity early which leads to possible limitation on the severity of ototoxicity.
Keywords
cisplatin ototoxicity - ototoxicity monitoring - dose dependent toxicity - ototoxicity - pure-tone audiometry - hearing lossIntroduction
According to the GLOBOCAN 2020 data, the total number of new cases of cancer was 19,292,789, and total mortality was 99,58,133.[1] Among them, the most common cancers diagnosed in females after breast cancer were head and neck, cervical, lung, and gastrointestinal cancers. These cancer types were observed to be common in males as well. The platinum-based chemotherapeutic agents, cisplatin and carboplatin, are two of the most widely used chemotherapeutic agents in these types of cancer.
Cisplatin has various dose limiting and cumulative toxicities. Ototoxicity is an important cumulative toxicity of cisplatin. This exclusively affects the cochlea. For cisplatin, hearing loss is bilateral, sensory neuronal, irreversible, and generally occurs at higher frequencies (>4 kHz) and is proportional to the cumulative dose of the drug.[2] Hearing loss is common at the higher frequencies when the cisplatin dose is greater than 60 mg/m2.[3] Often, it is accompanied by transient or permanent tinnitus which is commonly reversible on the completion of treatment.[4]
In literature, the rate of hearing loss is variably reported between 4 and 90%, depending on the drug dose, age of the patient, preexisting hearing loss, concurrent cranial radiation, some genetic factors, or concurrent use of other ototoxic medications.[5] A correlation between genetic variants and hearing loss has been found for cisplatin-detoxifying enzymes (glutathione-S-transferase),[6] nucleotide excision repair proteins, and megalin (low-density lipoprotein).[7]
Recently, sodium thiosulfate has been approved by the Food and Drug Administration (FDA) to prevent cisplatin-induced ototoxicity in children aged between 1 and 18 years.[8] Unfortunately, it is not yet approved for adult patients. The nature of ototoxicity is such that it often goes undetected until speech intelligibility is affected[9] and usually detected only when a communication problem becomes evident. These patients can be prescribed a hearing aid,[10] cochlear implant,[11] or other assistive device (text messaging or audio streamers), and other special accommodations which will optimize the quality of life for cancer survivors.
Early detection of ototoxicity is an essential component of cancer care in patients receiving cisplatin. Pure-tone audiometry (PTA) remains the first-line diagnostic tool for the screening, diagnosis, and follow-up of hearing status in these patients. So, by doing baseline and periodic audiometry, we can prevent administration of such ototoxic drug in patients of preexisting hearing loss and could detect cisplatin-induced sensory neuronal hearing loss (SNHL) early.
Therefore, this study aims to serve as a resource for health professionals to enhance their understanding of ototoxicity and its regular monitoring in patients receiving cisplatin to prevent ototoxicity. The primary aim of our study is to evaluate cisplatin-induced ototoxicity in cancer patients. Our objectives were to study the dose and effect relationship of cisplatin ototoxicity, severity and reversibility of cisplatin ototoxicity, and other associated risk factors enhancing cisplatin-associated ototoxicity.
Materials and Methods
This prospective study was performed among randomly selected 80 patients with diagnosed cancer and commencing treatment with cisplatin from August 2018 to July 2020 at a tertiary cancer health care center in western Gujarat. For estimation of sample size, the following formula was used:
n = [Zα 2 PQ] / d 2
Where:
Zα = value of standard normal variate corresponding to α level of significance = 1.96 (corresponding to 95% confidence interval).
P = likely value of parameter = 20%.
Q = 1–P = (100–20)% = 80%.
d = margin of errors (measure of precision) = 0.10 (10%).
Inclusion Criteria
Inclusion criteria are listed below:
Adults >18 years of age.
Positive diagnosis of cancer.
Commencing the first cycle of chemotherapy with cisplatin.
Patients on concurrent radiation with cisplatin were also included.
Exclusion Criteria
Exclusion criteria are as follows:
Patients presenting with profound hearing loss (more than 91 db) at baseline assessment, as it would be difficult to evaluate them according to the American Speech Language–Hearing Association criteria.[12]
Patients who have previously received cisplatin chemotherapy.
History of medical condition, such as tuberculosis and malaria (medications used in these conditions are ototoxic) or diabetes, heart failure, and renal failure medications, of which can affect the hearing threshold.
A total of 80 patients were randomly selected fulfilling the above inclusion criteria. Data collection was done in form of history, examination, complete blood count (CBC), biochemistry, biopsy of accessible site, imaging of relevant site, tumor markers, history regarding hearing loss, tinnitus, and pure-tone average at high frequency. PTA was performed at baseline frequency, at first, third, and sixth months (from beginning the first cycle of cisplatin) in both the ears. Patients were asked about symptoms like hearing loss, tinnitus, and vertigo with each PTA test. We have tested the hearing threshold in decibel (dB) from 250 to1,200 Hz for both the ears during PTA at multiple time period as discussed above. But for comparison of the hearing threshold in dB, we have used only 8-kHz frequency, as cisplatin-induced hearing loss occurs only at high frequency. The difference between hearing threshold (in dBHL) from the baseline audiogram and the posttreatment audiogram of all the patients were counted.
Significant hearing loss was considered in the following scenarios: approximately 20 Db decline in hearing observed at any single test frequency, oral 0-dB decline at two adjacent frequencies, or loss of response at maximum audiometer outputs for three consecutive frequencies where there was previously measurable hearing. This was based on the American Speech Language and Hearing Association criteria.[13] We graded severity of cisplatin-induced ototoxicity according to the World Health Organization (WHO) criteria.[14]
In primary outcome, we found that cisplatin ototoxicity is proportional to cumulative doses of drug. For secondary outcomes, we studied effect of age, sex, concomitant cranial radiation, chemotherapy given with cisplatin, baseline hearing loss, and cumulative dose. Among them, baseline hearing loss and cumulative doses of cisplatin, both being associated with ototoxicity, were found to be statistically significant.
Statistical Analysis
The data were entered in MS Excel spread sheet and analysis was done using Statistical Package for Social Sciences (SPSS) version 21.0. Quantitative variables were compared using paired t-test. Qualitative variables were analyzed using Chi-square test.
Ethics
The study was approved by the Institutional Ethics Committee clinical studies, Apollo Hospitals International Ltd., Ahmedabad, Gujarat, India. (approval no: ECR/30/INST/GJ/2013/RR; approved on July 16, 2018). The study was conducted according to the principles outlined in the International Conference on Harmonization Good Clinical Practice guidelines and in compliance with the protocol, the Data Protection Act and all other ethical and regulatory requirements, as appropriate for the study. All study participants were explained about study, and written informed consent was obtained from them. The Helsinki Declaration was followed which said that all procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Results
This study included 80 patients of cancer, commencing their treatment with first dose of cisplatin repeating either 1 or 3 weekly, given as concurrent with radiotherapy, as an adjuvant or as a neoadjuvant form with other chemotherapy agents or as a single agent.
We found that mean age of the patients in our study was 48.9 ± 10.11 years (range: 22–67 years; [Fig. 1]). Here, 70% (n = 56) of the patients were males and rest were women (30%, n = 24). The most common diagnosis for which cisplatin was prescribed was squamous cell carcinoma of head and neck (42.5%, n = 34). Treatment history of the patients revealed that 42.5% (n = 34) of the patients received cranial radiation therapy along with cisplatin. Gemcitabine, docetaxel with 5-fluorouracil, etoposide, bleomycin with etoposide, and pemetrexed were used with cisplatin, respectively, in 23.8 (n = 19), 10 (n = 8), 10 (n = 8), 7.5 (n = 6), and 6.3% (n = 5) of the patients.
| Figure 1:Distribution of patients according to age.
Of our study population, 32.5% (n = 26) received 0 to 200 mg/m2 cumulative dose of cisplatin, with median dose being 180 mg/m2. A total of 43.8% (n = 35) patients received 201 to 300 mg/m2 cumulative dose, with median dose of cisplatin being 290 mg/m2. About 23.8% (n = 19) of patients received 301 to 400 mg/m2 of cisplatin with a median dose being 320 mg/m2 ([Fig. 2]).
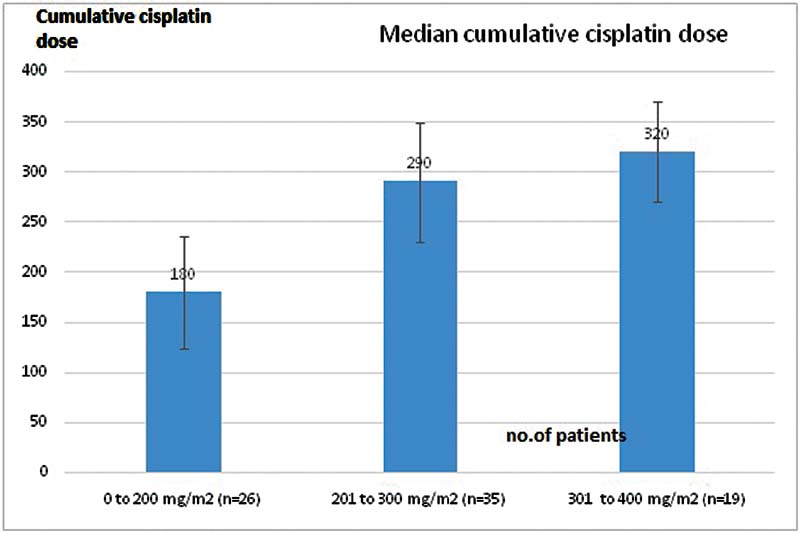
| Figure 2:Median dose of cisplatin given to the patients in the three groups.
At baseline, the mean PTA in right ear was 22.59 ± 4.03 dB which increased significantly to 32.89 ± 16.22 dB at 3 months (p < 0.001), and in left ear it was 22.59 ± 3.85 dB which increased significantly to 33.01 ± 16.04 dB at 3months (p < 0.001) ([Table 1]).
|
PTA (dB) |
Mean |
Standard deviation |
p-Value |
|
|---|---|---|---|---|
|
Right ear |
Baseline |
22.59 |
4.03 |
< 0.01 |
|
At 3 months |
32.89 |
16.22 |
||
|
Left ear |
Baseline |
22.59 |
3.85 |
< 0.001 |
|
At 3 months |
33.01 |
16.04 |
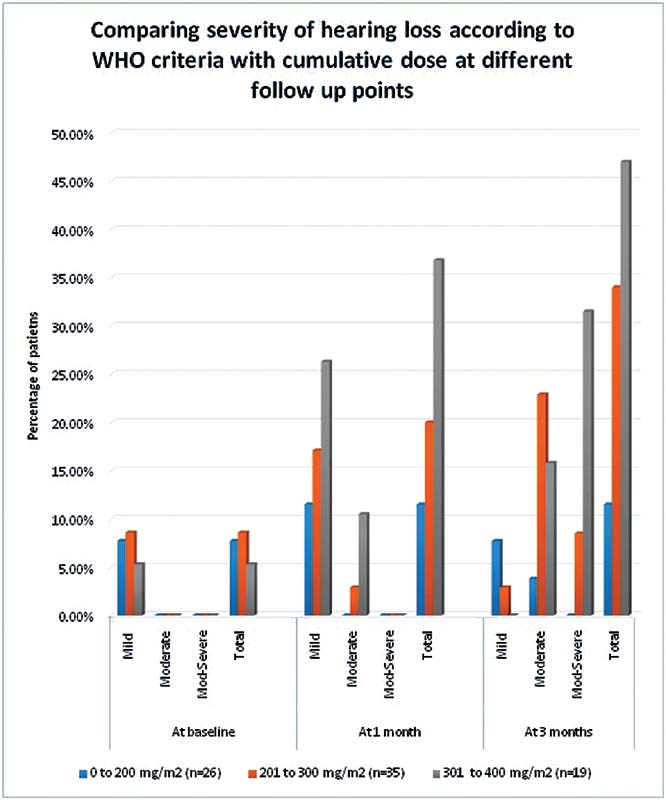
| Figure 3:Comparison of patients in different dose groups with the severity and type of hearing loss according to WHO grading. WHO, World Health Organization.
We found that subjective hearing loss was significantly more common in the 301 to 400 mg/m2 cumulative dose group (36.8%) as compared with patients in the other two-dose groups (p < 0.01). Tinnitus was not found to be significantly associated with the cumulative dose of cisplatin given to patients (p = 0.14), and it was reported by 8.8% (7 out of 80) of the patients.
We assessed the effects of age, gender, history of cranial radiation, chemotherapy given with cisplatin, baseline hearing loss, and cumulative cisplatin dose on ototoxicity. In multivariate step-wise regression analysis, baseline hearing loss (odds ratio [OR] = 17.71, 95% confidence interval [CI]: = 6.57–118.91, p < 0.05) and cumulative dose of cisplatin more than 300 mg/m2 (OR = 6.62, 95% CI: 2.33–18.74, p < 0.05) were found to be significantly associated with hearing loss at 3 months ([Table 2]).
|
Parameter |
Univariate analysis |
Multivariate analysis** |
||||
|---|---|---|---|---|---|---|
|
OR* |
95% CI |
p-Value |
OR |
95% CI |
p-Value |
|
|
Age (in y) |
||||||
|
Up to 50 |
Reference |
– |
– |
– |
– |
– |
|
> 50 |
1.82 |
0.67–4.91 |
0.23 |
1.82 |
0.54 to 6.09 |
0.33 |
|
Female gender |
1.12 |
0.39–3.25 |
0.94 |
– |
– |
– |
|
Crania radiation Received |
0.53 |
0.19–1.51 |
0.23 |
0.91 |
0.17–4.83 |
0.91 |
|
Concomitant chemotherapy given |
4.8 |
1.45–16 |
< 0.01 |
2.06 |
0.26–16.37 |
0.49 |
|
Baseline HL |
16.76 |
1.83–153.48 |
< 0.01 |
17.71 |
6.57–118.91 |
< 0.05 |
|
Cumulative cisplatin dose (mg/m2) |
||||||
|
0–200 |
Reference |
– |
– |
Reference |
– |
– |
|
201–300 |
3.51 |
0.86–14.23 |
0.07 |
4.13 |
0.67–15.23 |
0.12 |
|
301–400 |
5.57 |
1.23–25.21 |
< 0.05 |
6.62 |
2.33–18.74 |
< 0.05 |
References
- Sung H, Ferlay J, Siegel RL. et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2021; 71 (03) 209-249
- Simon T, Hero B, Dupuis W, Selle B, Berthold F. The incidence of hearing impairment after successful treatment of neuroblastoma. Klin Padiatr 2002; 214 (04) 149-152
- Rademaker-Lakhai JM, Crul M, Zuur L. et al. Relationship between cisplatin administration and the development of ototoxicity. J Clin Oncol 2006; 24 (06) 918-924
- Australasian Society of Clinical and Experimental Pharmacologists and Toxicologists; Pharmaceutical Society of Australia; Royal Australian College of General Practitioners. Australian Medicines Handbook. Adelaide, Australia: Pharmeutical Society of Australia; 2017
- Landier W. Ototoxicity and cancer therapy. Cancer 2016; 122 (11) 1647-1658
- Oldenburg J, Kraggerud SM, Cvancarova M, Lothe RA, Fossa SD. Cisplatin-induced long-term hearing impairment is associated with specific glutathione s-transferase genotypes in testicular cancer survivors. J Clin Oncol 2007; 25 (06) 708-714
- Riedemann L, Lanvers C, Deuster D. et al. Megalin genetic polymorphisms and individual sensitivity to the ototoxic effect of cisplatin. Pharmacogenomics J 2008; 8 (01) 23-28
- Brock PR, Maibach R, Childs M. et al. Sodium thiosulfate for protection from cisplatin-induced hearing loss. N Engl J Med 2018; 378 (25) 2376-2385
- Konrad-Martin D, Helt WJ, Reavis KM. et al. Ototoxicity: early detection and monitoring. ASHA Lead 2005; 10: 1-4
- Parsa V, Scollie S, Glista D, Seelisch A. Nonlinear frequency compression: effects on sound quality ratings of speech and music. Trends Amplif 2013; 17 (01) 54-68
- Durrant JD, Campbell K, Fausti S. et al. American Academy of Audiology: position statement and clinical practice guidelines: ototoxicity monitoring. Accessed July 26, 2022 at: https://audiology-web.s3.amazonaws.com/migrated/OtoMonGuidelines.pdf_539974c40999c1.58842217.pdf
- American Speech-Language-Hearing Association. Audiologic management of individuals receiving cochleotoxic drug therapy (guideline). Accessed July 26, 2022 at: https://www.asha.org/policy/gl1994-00003/
- American Speech-Language-Hearing Association. Audiologic management of individuals receiving cochleotoxic drug therapy. ASHA 1994; 36: 1-19
- World Health Organization. Report of the informal working group on prevention of deafness and hearing impairment programme planning. Accessed July 26, 2022 at: https://apps.who.int/iris/bitstream/handle/10665/58839/WHO_PDH_91.1.pdf?sequence=1&isAllowed=y
- Arslan E, Orzan E, Santarelli R. Global problem of drug-induced hearing loss. Ann N Y Acad Sci 1999; 884: 1-14
- Wang J, Lloyd Faulconbridge RV, Fetoni A, Guitton MJ, Pujol R, Puel JL. Local application of sodium thiosulfate prevents cisplatin-induced hearing loss in the guinea pig. Neuropharmacology 2003; 45 (03) 380-393
- Macdonald MR, Harrison RV, Wake M, Bliss B, Macdonald RE. Ototoxicity of carboplatin: comparing animal and clinical models at the Hospital for Sick Children. J Otolaryngol 1994; 23 (03) 151-159
- Gonçalves MS, Silveira AF, Teixeira AR, Hyppolito MA. Mechanisms of cisplatin ototoxicity: theoretical review. J Laryngol Otol 2013; 127 (06) 536-541
- Hellberg V, Wallin I, Ehrsson H, Laurell G. Cochlear pharmacokinetics of cisplatin: an in vivo study in the guinea pig. Laryngoscope 2013; 123 (12) 3172-3177
- Olgun Y. Cisplatin ototoxicity: where we are?. J Int Adv Otol 2013;9(03):
- Wang J, Puel JL, Bobbin R, Bobbin R, Puel JL. Mechanisms of toxicity in the cochlea (including physical free radical: oxidative and anti-oxidative mechanisms, protein interactions, and defense mechanisms). In: Campbell KC. Pharmacology and Ototoxicity for Audiologists. Clifton Park, NY: Delmar Cengage Learning; 2007: 70-81
- Dwivedi G, Kumar M, Gupta V, Sood A, Patnaik U. A clinical study of cisplatin induced ototoxicity in head and neck malignancies. Int J Otorhinolaryngol Head Neck Surg 2019; 5: 1044-1051
- Arora R, Thakur JS, Azad RK, Mohindroo NK, Sharma DR, Seam RK. Cisplatin-based chemotherapy: add high-frequency audiometry in the regimen. Indian J Cancer 2009; 46 (04) 311-317
- Dutta A, Venkatesh MD, Kashyap RC. Study of the effects of chemotherapy on auditory function. Indian J Otolaryngol Head Neck Surg 2005; 57 (03) 226-228
- Greene JB, Standring R, Siddiqui F, Ahsan SF. Incidence of cisplatin induced ototoxicity in adults with head and neck cancer. Advances in Otolaryngology 2015; 2015: 245613
- Bokemeyer C, Berger CC, Hartmann JT. et al. Analysis of risk factors for cisplatin-induced ototoxicity in patients with testicular cancer. Br J Cancer 1998; 77 (08) 1355-1362
- Frisina RD, Wheeler HE, Fossa SD. et al. Comprehensive audiometric analysis of hearing impairment and tinnitus after cisplatin-based chemotherapy in survivors of adult-onset cancer. J Clin Oncol 2016; 34 (23) 2712-2720
- Cho SI, Lee JE, Do NY. Protective effect of silymarin against cisplatin-induced ototoxicity. Int J Pediatr Otorhinolaryngol 2014; 78 (03) 474-478
- Whitehorn H, Sibanda M, Lacerda M. et al. High prevalence of cisplatin-induced ototoxicity in Cape Town, South Africa. S Afr Med J 2014; 104 (04) 288-291
- Skalleberg J, Solheim O, Fosså SD. et al. Long-term ototoxicity in women after cisplatin treatment for ovarian germ cell cancer. Gynecol Oncol 2017; 145 (01) 148-153
- Helson L, Okonkwo E, Anton L, Cvitkovic E. cis-Platinum ototoxicity. Clin Toxicol 1978; 13 (04) 469-478
- Yancey A, Harris MS, Egbelakin A, Gilbert J, Pisoni DB, Renbarger J. Risk factors for cisplatin-associated ototoxicity in pediatric oncology patients. Pediatr Blood Cancer 2012; 59 (01) 144-148
- Jacob LC, Aguiar FP, Tomiasi AA, Tschoeke SN, Bitencourt RF. Auditory monitoring in ototoxicity. Rev Bras Otorrinolaringol (Engl Ed) 2006; 72 (06) 836-844
- Yu KK, Choi CH, An YH. et al. Comparison of the effectiveness of monitoring Cisplatin-induced ototoxicity with extended high-frequency pure-tone audiometry or distortion-product otoacoustic emission. Korean J Audiol 2014; 18 (02) 58-68
Address for correspondence
Shirish S. Alurkar, MD MedicineDepartment of Medical Oncology, Apollo CBCC Cancer CareAhmadabad; Apollo International hospital Limited, GIDC estate, Bhat, Gandhinagar 382424, GujaratIndiaEmail: ssalurkar@gmail.comPublication History
Article published online:
20 October 2022© 2022. Indian Society of Medical and Paediatric Oncology. This is an open access article published by Thieme under the terms of the Creative Commons Attribution-NonDerivative-NonCommercial License, permitting copying and reproduction so long as the original work is given appropriate credit. Contents may not be used for commercial purposes, or adapted, remixed, transformed or built upon. (https://creativecommons.org/licenses/by-nc-nd/4.0/)
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India

| Figure 1:Distribution of patients according to age.

| Figure 2:Median dose of cisplatin given to the patients in the three groups.

| Figure 3:Comparison of patients in different dose groups with the severity and type of hearing loss according to WHO grading. WHO, World Health Organization.
References
- Sung H, Ferlay J, Siegel RL. et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2021; 71 (03) 209-249
- Simon T, Hero B, Dupuis W, Selle B, Berthold F. The incidence of hearing impairment after successful treatment of neuroblastoma. Klin Padiatr 2002; 214 (04) 149-152
- Rademaker-Lakhai JM, Crul M, Zuur L. et al. Relationship between cisplatin administration and the development of ototoxicity. J Clin Oncol 2006; 24 (06) 918-924
- Australasian Society of Clinical and Experimental Pharmacologists and Toxicologists; Pharmaceutical Society of Australia; Royal Australian College of General Practitioners. Australian Medicines Handbook. Adelaide, Australia: Pharmeutical Society of Australia; 2017
- Landier W. Ototoxicity and cancer therapy. Cancer 2016; 122 (11) 1647-1658
- Oldenburg J, Kraggerud SM, Cvancarova M, Lothe RA, Fossa SD. Cisplatin-induced long-term hearing impairment is associated with specific glutathione s-transferase genotypes in testicular cancer survivors. J Clin Oncol 2007; 25 (06) 708-714
- Riedemann L, Lanvers C, Deuster D. et al. Megalin genetic polymorphisms and individual sensitivity to the ototoxic effect of cisplatin. Pharmacogenomics J 2008; 8 (01) 23-28
- Brock PR, Maibach R, Childs M. et al. Sodium thiosulfate for protection from cisplatin-induced hearing loss. N Engl J Med 2018; 378 (25) 2376-2385
- Konrad-Martin D, Helt WJ, Reavis KM. et al. Ototoxicity: early detection and monitoring. ASHA Lead 2005; 10: 1-4
- Parsa V, Scollie S, Glista D, Seelisch A. Nonlinear frequency compression: effects on sound quality ratings of speech and music. Trends Amplif 2013; 17 (01) 54-68
- Durrant JD, Campbell K, Fausti S. et al. American Academy of Audiology: position statement and clinical practice guidelines: ototoxicity monitoring. Accessed July 26, 2022 at: https://audiology-web.s3.amazonaws.com/migrated/OtoMonGuidelines.pdf_539974c40999c1.58842217.pdf
- American Speech-Language-Hearing Association. Audiologic management of individuals receiving cochleotoxic drug therapy (guideline). Accessed July 26, 2022 at: https://www.asha.org/policy/gl1994-00003/
- American Speech-Language-Hearing Association. Audiologic management of individuals receiving cochleotoxic drug therapy. ASHA 1994; 36: 1-19
- World Health Organization. Report of the informal working group on prevention of deafness and hearing impairment programme planning. Accessed July 26, 2022 at: https://apps.who.int/iris/bitstream/handle/10665/58839/WHO_PDH_91.1.pdf?sequence=1&isAllowed=y
- Arslan E, Orzan E, Santarelli R. Global problem of drug-induced hearing loss. Ann N Y Acad Sci 1999; 884: 1-14
- Wang J, Lloyd Faulconbridge RV, Fetoni A, Guitton MJ, Pujol R, Puel JL. Local application of sodium thiosulfate prevents cisplatin-induced hearing loss in the guinea pig. Neuropharmacology 2003; 45 (03) 380-393
- Macdonald MR, Harrison RV, Wake M, Bliss B, Macdonald RE. Ototoxicity of carboplatin: comparing animal and clinical models at the Hospital for Sick Children. J Otolaryngol 1994; 23 (03) 151-159
- Gonçalves MS, Silveira AF, Teixeira AR, Hyppolito MA. Mechanisms of cisplatin ototoxicity: theoretical review. J Laryngol Otol 2013; 127 (06) 536-541
- Hellberg V, Wallin I, Ehrsson H, Laurell G. Cochlear pharmacokinetics of cisplatin: an in vivo study in the guinea pig. Laryngoscope 2013; 123 (12) 3172-3177
- Olgun Y. Cisplatin ototoxicity: where we are?. J Int Adv Otol 2013;9(03):
- Wang J, Puel JL, Bobbin R, Bobbin R, Puel JL. Mechanisms of toxicity in the cochlea (including physical free radical: oxidative and anti-oxidative mechanisms, protein interactions, and defense mechanisms). In: Campbell KC. Pharmacology and Ototoxicity for Audiologists. Clifton Park, NY: Delmar Cengage Learning; 2007: 70-81
- Dwivedi G, Kumar M, Gupta V, Sood A, Patnaik U. A clinical study of cisplatin induced ototoxicity in head and neck malignancies. Int J Otorhinolaryngol Head Neck Surg 2019; 5: 1044-1051
- Arora R, Thakur JS, Azad RK, Mohindroo NK, Sharma DR, Seam RK. Cisplatin-based chemotherapy: add high-frequency audiometry in the regimen. Indian J Cancer 2009; 46 (04) 311-317
- Dutta A, Venkatesh MD, Kashyap RC. Study of the effects of chemotherapy on auditory function. Indian J Otolaryngol Head Neck Surg 2005; 57 (03) 226-228
- Greene JB, Standring R, Siddiqui F, Ahsan SF. Incidence of cisplatin induced ototoxicity in adults with head and neck cancer. Advances in Otolaryngology 2015; 2015: 245613
- Bokemeyer C, Berger CC, Hartmann JT. et al. Analysis of risk factors for cisplatin-induced ototoxicity in patients with testicular cancer. Br J Cancer 1998; 77 (08) 1355-1362
- Frisina RD, Wheeler HE, Fossa SD. et al. Comprehensive audiometric analysis of hearing impairment and tinnitus after cisplatin-based chemotherapy in survivors of adult-onset cancer. J Clin Oncol 2016; 34 (23) 2712-2720
- Cho SI, Lee JE, Do NY. Protective effect of silymarin against cisplatin-induced ototoxicity. Int J Pediatr Otorhinolaryngol 2014; 78 (03) 474-478
- Whitehorn H, Sibanda M, Lacerda M. et al. High prevalence of cisplatin-induced ototoxicity in Cape Town, South Africa. S Afr Med J 2014; 104 (04) 288-291
- Skalleberg J, Solheim O, Fosså SD. et al. Long-term ototoxicity in women after cisplatin treatment for ovarian germ cell cancer. Gynecol Oncol 2017; 145 (01) 148-153
- Helson L, Okonkwo E, Anton L, Cvitkovic E. cis-Platinum ototoxicity. Clin Toxicol 1978; 13 (04) 469-478
- Yancey A, Harris MS, Egbelakin A, Gilbert J, Pisoni DB, Renbarger J. Risk factors for cisplatin-associated ototoxicity in pediatric oncology patients. Pediatr Blood Cancer 2012; 59 (01) 144-148
- Jacob LC, Aguiar FP, Tomiasi AA, Tschoeke SN, Bitencourt RF. Auditory monitoring in ototoxicity. Rev Bras Otorrinolaringol (Engl Ed) 2006; 72 (06) 836-844
- Yu KK, Choi CH, An YH. et al. Comparison of the effectiveness of monitoring Cisplatin-induced ototoxicity with extended high-frequency pure-tone audiometry or distortion-product otoacoustic emission. Korean J Audiol 2014; 18 (02) 58-68


 PDF
PDF  Views
Views  Share
Share

