Prosthetic Management of Hard Palate Perforation in a Child with Acute Lymphoblastic Leukemia
CC BY-NC-ND 4.0 · Indian J Med Paediatr Oncol 2017; 38(02): 220-222
DOI: DOI: 10.4103/ijmpo.ijmpo_17_17
Abstract
Palatal perforation is an uncommon complication seen in children with acute lymphoblastic leukemia undergoing chemotherapy. This may impact basic functions, such as speech, swallowing, chewing, affecting the quality of life (QOL). Prosthetic rehabilitation of the palatal perforation with obturator can optimally restore function, thereby improving and enhancing the QOL of these patients.
Publication History
Article published online:
06 July 2021
© 2017. Indian Society of Medical and Paediatric Oncology. This is an open access article published by Thieme under the terms of the Creative Commons Attribution-NonDerivative-NonCommercial-License, permitting copying and reproduction so long as the original work is given appropriate credit. Contents may not be used for commercial purposes, or adapted, remixed, transformed or built upon. (https://creativecommons.org/licenses/by-nc-nd/4.0/.)
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India
Abstract
Palatal perforation is an uncommon complication seen in children with acute lymphoblastic leukemia undergoing chemotherapy. This may impact basic functions, such as speech, swallowing, chewing, affecting the quality of life (QOL). Prosthetic rehabilitation of the palatal perforation with obturator can optimally restore function, thereby improving and enhancing the QOL of these patients.
Introduction
Children with leukemia have impaired immunity and are more prone for fungal infection. The infection usually involves the nasal cavity, maxilla, paranasal sinuses, and orbits. Necrosis of the palate is often seen when the fungus invades the arteries, forms thrombi within the blood vessels that reduce the blood supply.[1] Fungal pathogens are subdivided into those that remain superficial (i.e., restricted to the epithelial surface) and those that invade deep organs and tissues (deep fungi). Invasion of surrounding tissue can cause necrotizing the ulceration of palate with a blackish slough and exposure of bone.[2]
Treatment may require resection of the palate, maxilla, and facial tissues to remove the extent of necrosis. If the patient survives, the resulting defect needs prosthesis to close the defect which is termed as a maxillary obturator. An obturator (Latin: Obturare, to stop up) is a disc or plate, natural, or artificial, which closes an opening or defect of the maxilla as a result of a cleft palate or partial or total removal of the maxilla for a tumor mass.[3]
The goals of prosthetic rehabilitation include separation of oral and nasal cavities which helps to improve the basic functions, such as speech, swallowing, chewing.[4] These defects are more difficult to restore because they are generally lined with respiratory mucosa and poorly keratinized squamous epithelium, making it more difficult for the patient to tolerate the prosthesis.[5]
This case report describes prosthodontic management of a pediatric patient who developed a palatal perforation following chemotherapy for acute lymphoblastic leukemia (ALL).
Case Report
A 6-year-old boy, case of ALL, was referred for prosthetic rehabilitation of a palatal perforation following chemotherapy [Figure 1]. A detailed case history revealed that the patient was diagnosed with B-cell ALL at the age of 5 years and was on consolidation chemotherapy. Palatal biopsy showed hyperplastic ulcerated squamous epithelium admixed with scanty bony bits and necroinflammatory cells with underlying granulation tissue. Ulcer slough contained a few fungal hyphae suggestive of secondary colonization. Noncontrast computerized tomography scan revealed mucosal pansinusitis and rhinitis. Nasal secretion test for fungal identification and susceptibility testing showed the presence of Candida tropicalis. On oral examination, a 2 cm × 2 cm perforation was seen on the left side of the hard palate with slough. It was decided to make a palatal obturator to cover the palatal perforation. This defect in the palate led to difficulty in eating, swallowing, and nasal regurgitation. Speech too was hypernasal and not intelligible. It was decided to cover the palatal perforation by fabrication of a palatal obturator.
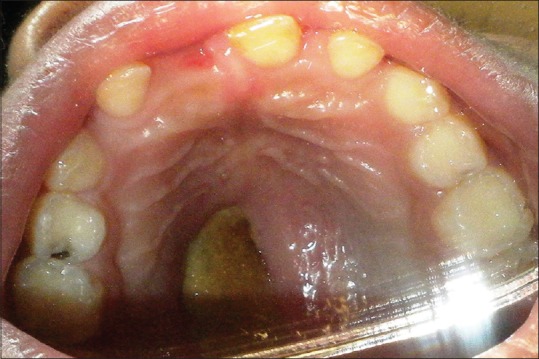
| Figure 1:Intraoral view showing the palatal perforation
A stainless steel stock dentulous tray and irreversible hydrocolloid (Dentalgin; Prime Dental Products, Mumbai, India) were used to record preliminary impression of the maxillary arch [Figure 2]. Mandibular impression was also made with irreversible hydrocolloid. Stone (Kaldent Dental Stone Class III, Kalabhai Karson Pvt. Ltd.) working cast were obtained from the impression. A 19-gauge hard, round, stainless steel orthodontic wire (KC Smith and Co., Monmouth, UK) was used to fabricate the full wire clasp and C clasps for the palatal obturator as shown in figure. A single thickness modeling wax was adapted on the maxillary cast covering entire hard palate and subsequently acrylized into the heat-polymerized acrylic resin (DPI Dentsply, India). The prosthesis was finished and polished in usual manner [Figure 3]. The prosthesis was delivered, and postinsertion instructions were given to the patient regarding hygiene maintenance in the oral cavity [Figure 4]. It was observed that the patient was very comfortable using this removable prosthesis which obturated the palatal defect. This palatal obturator facilitated eating and swallowing. The patient could also comfortably communicate as the speech could be understood by the listeners. The patient was followed up at regular interval. This option of prosthetic obturation of the palatal defect was preferred over surgical closure in view of the medical history and general condition of the patient.
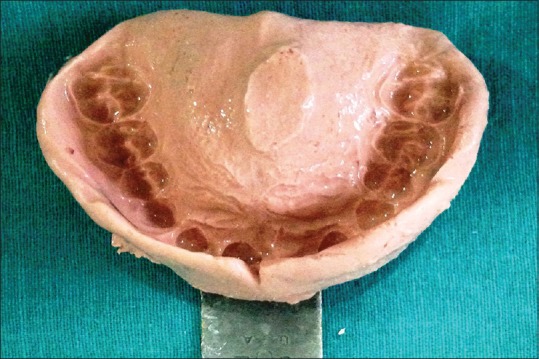
| Figure 2:Impression of the maxillary arch
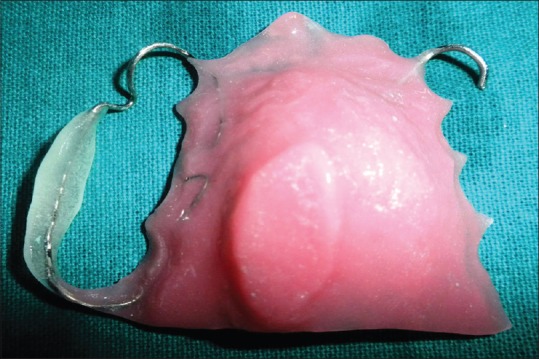
| Figure 3:Finished prosthesis
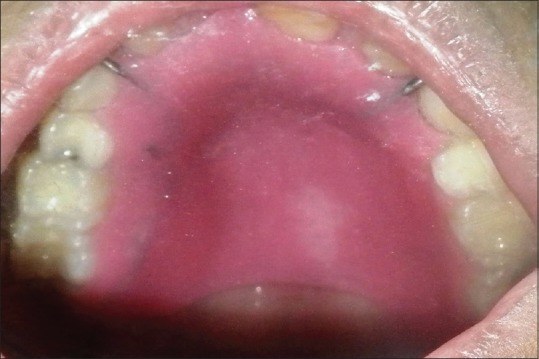
| Figure 4:Prosthesis insertion done
Discussion
Leukemia is a malignancy affecting the white blood cells of the bone marrow. Virchow in 1874 described leukemia as “white blood.”[6] ALL is more common among children and accounts for 75% of childhood acute leukemia.[7]
Palate perforation can occur due to trauma, bacterial infection such as osteomyelitis, Wegener's granulomatosis, viral infection such as herpes zoster, fungal infection such as mucormycosis, aspergillosis, prolonged cocaine abuse, and malignancies.[8]
Palatal perforation is more common in immunocompromised patients as they are more prone to opportunistic infections that may invade the palate and cause palatal perforation.[9] This may predispose the patient to hypernasal speech, fluid leakage into the nasal cavity, and impaired masticator function. This severely affects the patient's quality of life (QOL).
Prosthetic rehabilitation of the palatal perforation with obturator by a maxillofacial prosthodontist has two primary objectives:[10]
- To optimally restore the functions of mastication, deglutition, and speech thereby improving and enhancing the QOL of these patients
- To achieve normal oro-facial appearance.[11]
Recall for the patient is usually done every 2 weeks due to rapid soft-tissue changes that occur within the defect during organization and healing of the wound.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Pogrel MA, Miller CE. A case of maxillary necrosis. J Oral Maxillofac Surg 2003;61:489-93.
- Rathi M, Goyal M, Kundu R. Prosthetic management of mucormycosis with palatal perforation: A case report. Int J Sci Res 2013;2:370-1.
- Chalian VA, Drane JB, Standish SM. Maxillofacial Prosthetics: Multidisciplinary Practice. Baltimore: The Williams and Wilkins Co.; 1971. p. 133-48.
- Kurrasch M, Beumer J 3rd, Kagawa T. Mucormycosis: Oral and prosthodontic implications. A report of 14 patients. J Prosthet Dent 1982;47:422-9.
- Beumer J 3rd, Marunick MT, Esposite SJ. Maxillofacial Rehabilitation: Prosthodontic and Surgical Management of Cancer Related, Acquired, and Congenital Defects of the Head and Neck. 3rd ed. Quintessence Publishing Co Inc. U.S.; 2011. p. 161.
- Greenberg MS, Glick M. Burket's Oral Medicine: Diagnosis and Treatment. 10th ed. BC Decker; Hamilton, Ontario; 2002. p. 443-5.
- Esparza SD, Sakamoto KM. Topics in pediatric leukemia – Acute lymphoblastic leukemia. MedGenMed 2005;7:23.
- Samanta DR, Senapati SN, Sharma PK, Shruthi BS, Paty PB, Sarangi G. Hard palate perforation in acute lymphoblastic leukemia due to mucormycosis – A case report. Indian J Hematol Blood Transfus 2009;25:36-9.
- Karabulut AB, Kabakas F, Berköz O, Karakas Z, Kesim SN. Hard palate perforation due to invasive aspergillosis in a patient with acute lymphoblastic leukemia. Int J Pediatr Otorhinolaryngol 2005;69:1395-8.
- Keyf F. Obturator prostheses for hemimaxillectomy patients. J Oral Rehabil 2001;28:821-9.
- Beumer J 3rd, Curtis TA, Firtell DN. Maxillofacial Rehabilitation: Prosthodontic and Surgical Considerations. St. Louis, Toronto, London: The C.V. Mosby Co.; 1979. p. 188-243.

| Figure 1:Intraoral view showing the palatal perforation

| Figure 2:Impression of the maxillary arch

| Figure 3:Finished prosthesis

| Figure 4:Prosthesis insertion done
References
- Pogrel MA, Miller CE. A case of maxillary necrosis. J Oral Maxillofac Surg 2003;61:489-93.
- Rathi M, Goyal M, Kundu R. Prosthetic management of mucormycosis with palatal perforation: A case report. Int J Sci Res 2013;2:370-1.
- Chalian VA, Drane JB, Standish SM. Maxillofacial Prosthetics: Multidisciplinary Practice. Baltimore: The Williams and Wilkins Co.; 1971. p. 133-48.
- Kurrasch M, Beumer J 3rd, Kagawa T. Mucormycosis: Oral and prosthodontic implications. A report of 14 patients. J Prosthet Dent 1982;47:422-9.
- Beumer J 3rd, Marunick MT, Esposite SJ. Maxillofacial Rehabilitation: Prosthodontic and Surgical Management of Cancer Related, Acquired, and Congenital Defects of the Head and Neck. 3rd ed. Quintessence Publishing Co Inc. U.S.; 2011. p. 161.
- Greenberg MS, Glick M. Burket's Oral Medicine: Diagnosis and Treatment. 10th ed. BC Decker; Hamilton, Ontario; 2002. p. 443-5.
- Esparza SD, Sakamoto KM. Topics in pediatric leukemia – Acute lymphoblastic leukemia. MedGenMed 2005;7:23.
- Samanta DR, Senapati SN, Sharma PK, Shruthi BS, Paty PB, Sarangi G. Hard palate perforation in acute lymphoblastic leukemia due to mucormycosis – A case report. Indian J Hematol Blood Transfus 2009;25:36-9.
- Karabulut AB, Kabakas F, Berköz O, Karakas Z, Kesim SN. Hard palate perforation due to invasive aspergillosis in a patient with acute lymphoblastic leukemia. Int J Pediatr Otorhinolaryngol 2005;69:1395-8.
- Keyf F. Obturator prostheses for hemimaxillectomy patients. J Oral Rehabil 2001;28:821-9.
- Beumer J 3rd, Curtis TA, Firtell DN. Maxillofacial Rehabilitation: Prosthodontic and Surgical Considerations. St. Louis, Toronto, London: The C.V. Mosby Co.; 1979. p. 188-243.


 PDF
PDF  Views
Views  Share
Share

