Pulmonary Aspergillosis Silently Presenting as Pneumothorax in Children with Leukemia: A Report of Three Cases.
CC BY-NC-ND 4.0 · Indian J Med Paediatr Oncol 2022; 43(05): 439-442
DOI: DOI: 10.1055/s-0042-1755545
Abstract
Aspergillosis causes invasive pulmonary disease in patients with hematological malignancies. Children with invasive pulmonary aspergillosis (IPA) usually have nonspecific radiographic findings unlike cavitary lesions commonly seen in adults. Pneumothorax due to rupture of peripheral fungal lesion may be a severe complication in patients with neutropenia. Here, we describe three children during induction chemotherapy for B-lymphoblastic leukemia with pneumothorax as a presenting feature of pulmonary aspergillosis.
Note
The manuscript has been read and approved by all the authors, and the requirements for authorship have been met, and each author believes that the manuscript represents honest work.
Declaration of Patient Consent
The authors certify that they have obtained all appropriate patient consent forms.
Publication History
Article published online:
20 October 2022
© 2022. Indian Society of Medical and Paediatric Oncology. This is an open access article published by Thieme under the terms of the Creative Commons Attribution-NonDerivative-NonCommercial License, permitting copying and reproduction so long as the original work is given appropriate credit. Contents may not be used for commercial purposes, or adapted, remixed, transformed or built upon. (https://creativecommons.org/licenses/by-nc-nd/4.0/)
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India
Abstract
Aspergillosis causes invasive pulmonary disease in patients with hematological malignancies. Children with invasive pulmonary aspergillosis (IPA) usually have nonspecific radiographic findings unlike cavitary lesions commonly seen in adults. Pneumothorax due to rupture of peripheral fungal lesion may be a severe complication in patients with neutropenia. Here, we describe three children during induction chemotherapy for B-lymphoblastic leukemia with pneumothorax as a presenting feature of pulmonary aspergillosis.
Introduction
Invasive pulmonary aspergillosis (IPA) is a potentially fatal opportunistic infection in patients with hematological malignancies and stem cell transplant recipients.[1] [2] Acute necrotizing bronchopneumonia, hemorrhagic pulmonary infarction, and lung abscess have been described in a large retrospective review of culture documented aspergillus infection in pediatric cancer patients but none of them manifested with pneumothorax.[3] We describe our observations of three children with B-lymphoblastic leukemia (B-ALL) who developed spontaneous pneumothorax that represented the first sign of IPA.
Case Description
Case 1
A 7-year-old male presented with fever, pallor, hepatomegaly, and splenomegaly. He was diagnosed with B-ALL and started on remission induction chemotherapy as per the Indian Childhood Collaborative Leukemia Group (ICiCLe) protocol under the intermediate-risk arm.[4] On day 22 of induction, child complained of difficulty in breathing and chest pain on left side. On physical assessment, the trachea was shifted to the right and hyperresonant percussion note on left hemithorax. Chest radiography showed lucent area in left lung field with absent bronchovesicular marking suggesting a pneumothorax with tracheal shift to right ([Fig. 1]). An emergent chest tube thoracostomy was performed. Contrast-enhanced computed tomography (CECT) showed bilateral patchy areas of consolidation involving left lower lobe, left upper lobe, and right lower lobe.
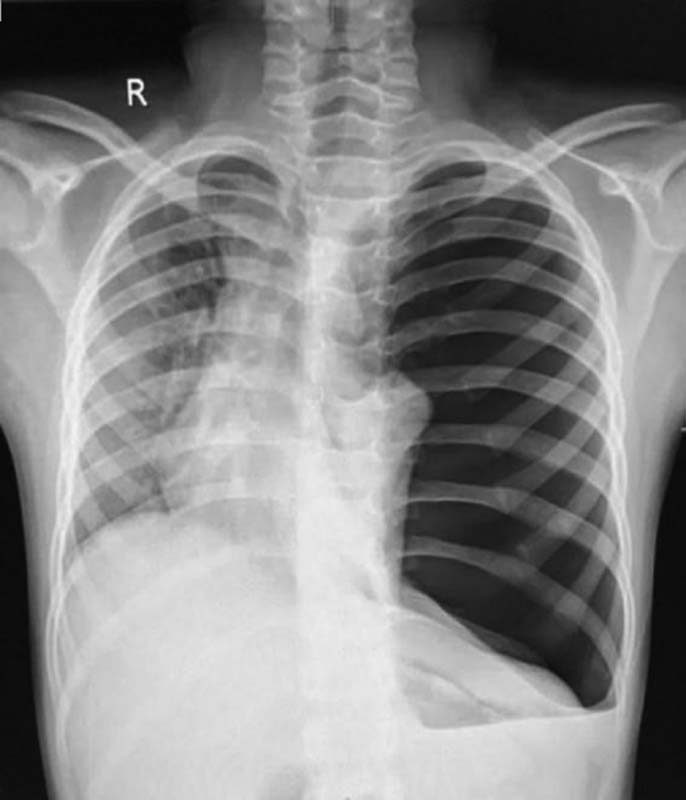
| Figure 1:Pneumothorax with tracheal shift to right
Serum galactomannan (GM) level was analyzed by immune-enzymatic sandwich microplate assay and was found to be optical density index of 0.85. Any value of >0.5 optical density index is considered positive by the assay. Blood culture was negative. The patchy consolidation resolved after 6 weeks of amphotericin B deoxycholate therapy at dose of 1 mg/kg/day once daily. In retrospect, it was noticed that there was rapid recovery of neutrophil counts from 500 to 1,500 cells/µL within 48 hours when child developed pneumothorax. At the end of induction, bone marrow was in remission and minimal residual disease was <0>
Case 2
A 9-year-old female presented with fever and easy fatigability since 4 weeks. Physical examination revealed pallor; cervical, axillary, and epitrochlear lymphadenopathy; hepatomegaly ; and splenomegaly. The child was diagnosed with B-ALL having hyperdiploidy and chromosome 6q deletion. Remission induction chemotherapy was started as per the ICiCLe protocol under the intermediate-risk arm. Second week of induction was complicated by reversible posterior leukoencephalopathy syndrome (RPLS). On day 30 of induction, child developed pleuritic chest pain on the right side. Chest radiograph suggested right pneumothorax which was confirmed on CECT thorax. There were multiple ill-defined heterogeneously enhancing nodules of varying size in bilateral lung fields in random distribution. Few cavitary lung lesions were observed in the anterior basal segment of right lower lobe. The child was started on amphotericin B deoxycholate with suspicion of invasive fungal infection. There was a recent neutrophil count recovery from 600 to 1,100 cells/µL when child had developed pneumothorax. Two days later, a small subcutaneous swelling was noted on anterior abdominal wall in the left iliac region. It was firm and tender on examination. The specimen obtained by needle aspiration was sent for microbiological evaluation which revealed septate fungal elements. Serum GM was positive with an index value of 2.11. There was modest improvement on CECT thorax after 3 weeks of therapy, with persistence of lung nodules in bilateral lung fields. Pneumothorax resolved spontaneously. Serum GM was negative (optical density index of 0.30) after 6 weeks of amphotericin B. Despite a challenging course of remission induction complicated by RPLS and invasive aspergillosis (IA) with pneumothorax, the child was able to achieve a post induction minimal residual disease of <0>
Case 3
A 9-year-old male with fever since 2 weeks had pallor, cervical lymphadenopathy, and oraganomegaly. On evaluation, child was diagnosed with B-ALL with hyperdiploidy and started on induction chemotherapy as per the ICiCLe protocol. On day 28, the child developed right-sided chest pain on deep inspiration. There were decreased chest excursions and diminished breath sounds on right hemithorax. Chest radiography showed collapse of right lung with lucent area in peripheral lung field with absent bronchovesicular marking suggesting a pneumothorax. Needle decompression of pleural space was performed followed by chest tube thoracostomy. CECT thorax showed patchy areas of consolidation in right lower lobe. Serum GM assay was positive. Child responded well after 3 weeks of amphotericin B dosage and achieved remission at the end of induction.
Discussion
The incidence of invasive fungal infections (IFIs) has increased over the last few years along with the increased number of immunocompromised patients.[5] Timely diagnosis and prompt initiation of antifungal treatment are crucial for care.[6] Spontaneous pneumothorax in children receiving remission induction chemotherapy for leukemia is seldom encountered. Pneumothorax occurs in approximately 13% of adults who have pulmonary fungal infection while receiving chemotherapy for hematological malignancies. But the incidence of pneumothorax due to IPA in pediatric age is not known, since it is exceedingly rare.
IPA is categorized as “proven,” “probable,” and “possible” based on clinical, microbiological, and radiological criteria as per the revised European Organization for Research and Treatment of Cancer and the Mycoses Study Group Education and Research Consortium (EORTC/MSGERC) consensus definition.[7] In this report, we describe pneumothorax as the first manifestation in one “proven (case 2)” and two “probable (cases 1 and 3)” cases of IPA. Bacterial blood cultures were negative, and fungal blood cultures were sent for testing but showed no growth in all the three cases.
Bronchoalveolar lavage could not be performed in patients due to prevailing clinical conditions and resource constraints. All the patients were on fluconazole for primary antifungal prophylaxis since beginning of the induction therapy. Voriconazole was continued as secondary prophylaxis after discontinuation of amphotericin B. Steroids were continued at second or third dose, and further induction was resumed once patients were clinically stable along with antifungal therapy.
Opportunistic infections during the early phase of leukemia treatment are a two-fold blow. They not only hamper the timely delivery of cytotoxic agents but are also difficult to treat and often lethal. The German ALL–Berlin–Frankfurt–Muenster (BFM) study group reported that fungal infections were responsible for one-fifth of fatal infections in pediatric ALL patients.[8]
Aspergillus was implicated in two-thirds of those with invasive fungal infection.[8] Corticosteroids and prolonged neutropenia are known risk factors for this.[9] Pulmonary aspergillosis presents with fever, pleuritic chest pain, and hemoptysis. Corticosteroids may mask the common presenting symptoms of IPA, mainly fever and cough. In a large survey of patients with IPA, typical manifestations were significantly less common in patients receiving steroids.[10] The occurrence of spontaneous pneumothorax may be the first manifestation of subpleural fungal infection in patients with leukemia. Poor nutrition, presence of subpleural lesion, bouts of excessive coughing, and prolonged corticosteroid therapy are various precipitating factors. Pleural lesions were seen in cases 2 and 3.
In review of radiological findings in children with IPA consolidation, perihilar infiltrates, multiple small nodules, peripheral nodular masses, and pleural effusions have been described but no findings have been suggestive of pneumothorax.[11] In a multicenter retrospective analysis of pediatric invasive pulmonary aspergillosis, the most common diagnostic radiologic finding was of nodules.[12] Children with proven IPA commonly have nonspecific changes on CECT compared with halo sign, air crescent formation, or cavitation seen in adults.[8] Rare possible complications of pulmonary aspergillosis include bronchopericardial fistula, pericarditis, tension pneumopericardium, and pericardial tamponade.[13]
A temporal correlation between neutrophil count recovery and occurrence of pneumothorax in patients with invasive fungal pneumonia has been demonstrated.[14] The neutrophils are attracted to site of infection by chemotactic factors. Along with its microbicidal activity, neutrophils release reactive oxygen intermediates and proteolytic enzymes like collagenase and elastase. These cytolytic molecules cause collateral damage by destruction of surrounding pulmonary parenchyma. This might explain the paradoxical clinical deterioration with neutrophil recovery observed in two of the cases (1 and 2) we described.
Early suspicion of aspergillosis helped us to start appropriate antifungal agent early, with favorable outcome during a crucial period of remission induction. The advent of biomarker tests such as the GM enzyme immunoassay (GM-EIA) provides a potential adjunct for noninvasive diagnosis of IA.[15] A limitation to our approach was that histopathologic or cytopathologic evidence of mold could not be proven in cases 1 and 3. Bronchoalveolar lavage and fungal DNA polymerase chain reaction (PCR) could not be performed due to resource constraints.
Conclusion
Our observation helps us to be vigilant for sudden deterioration and pneumothorax in children with ALL on induction therapy. A possibility of IPA should be considered when encountered with pneumothorax in children with leukemia on intensive chemotherapy. Based on clinical and radiological observations, recent recovery of neutrophil counts and presence of subpleural or peripheral lesion may increase the likelihood of pneumothorax.
Conflict of Interest
None declared.
Note
The manuscript has been read and approved by all the authors, and the requirements for authorship have been met, and each author believes that the manuscript represents honest work.
Declaration of Patient Consent
The authors certify that they have obtained all appropriate patient consent forms.
References
- Groll AH, Pana D, Lanternier F. et al; 8th European Conference on Infections in Leukaemia. 8th European Conference on Infections in Leukaemia: 2020 guidelines for the diagnosis, prevention, and treatment of invasive fungal diseases in paediatric patients with cancer or post-haematopoietic cell transplantation. Lancet Oncol 2021; 22 (06) e254-e269
- Pappas PG, Alexander BD, Andes DR. et al. Invasive fungal infections among organ transplant recipients: results of the Transplant-Associated Infection Surveillance Network (TRANSNET). Clin Infect Dis 2010; 50 (08) 1101-1111
- Abbasi S, Shenep JL, Hughes WT, Flynn PM. Aspergillosis in children with cancer: A 34-year experience. Clin Infect Dis 1999; 29 (05) 1210-1219
- Das N, Banavali S, Bakhshi S. et al. Protocol for ICiCLe-ALL-14 (InPOG-ALL-15-01): a prospective, risk stratified, randomised, multicentre, open label, controlled therapeutic trial for newly diagnosed childhood acute lymphoblastic leukaemia in India. Trials 2022; 23 (01) 102
- Pana ZD, Roilides E, Warris A, Groll AH, Zaoutis T. Epidemiology of invasive fungal disease in children. J Pediatric Infect Dis Soc 2017; 6 (suppl_1): S3-S11
- Tragiannidis A, Kattamis A, Vyzantiadis TA. Invasive fungal infections in children with haematological malignancies: diagnostic and therapeutic challenges. J Fungi (Basel) 2021; 7 (07) 516
- Donnelly JP, Chen SC, Kauffman CA. et al. Revision and update of the consensus definitions of invasive fungal disease from the European Organization for research and Treatment of cancer and the Mycoses Study Group education and research consortium. Clin Infect Dis 2020; 71 (06) 1367-1376
- Grigull L, Beier R, Schrauder A. et al. Invasive fungal infections are responsible for one-fifth of the infectious deaths in children with ALL. Mycoses 2003; 46 (11,12): 441-446
- Denning DW, Marinus A, Cohen J. et al; EORTC Invasive Fungal Infections Cooperative Group. An EORTC multicentre prospective survey of invasive aspergillosis in haematological patients: diagnosis and therapeutic outcome. J Infect 1998; 37 (02) 173-180
- Cornillet A, Camus C, Nimubona S. et al. Comparison of epidemiological, clinical, and biological features of invasive aspergillosis in neutropenic and nonneutropenic patients: a 6-year survey. Clin Infect Dis 2006; 43 (05) 577-584
- Thomas KE, Owens CM, Veys PA, Novelli V, Costoli V. The radiological spectrum of invasive aspergillosis in children: a 10-year review. Pediatr Radiol 2003; 33 (07) 453-460
- Burgos A, Zaoutis TE, Dvorak CC. et al. Pediatric invasive aspergillosis: a multicenter retrospective analysis of 139 contemporary cases. Pediatrics 2008; 121 (05) e1286-e1294
- Ödev K, Çaliskan U, Emlik D, Koç H, Koç S. Pneumomediastinum and pneumopericardium due to intracavitary aspergilloma: an unusual complication of fungal pneumonia. Pediatr Radiol 2002; 32 (02) 143-145
- Todeschini G, Murari C, Bonesi R. et al. Invasive aspergillosis in neutropenic patients: rapid neutrophil recovery is a risk factor for severe pulmonary complications. Eur J Clin Invest 1999; 29 (05) 453-457
- Huppler AR, Fisher
BT, Lehrnbecher T, Walsh TJ, Steinbach WJ. Role of molecular
biomarkers in the diagnosis of invasive fungal diseases in children. J Pediatric Infect Dis Soc 2017; 6
(suppl_1): S32-S44
Address for correspondence
Krunal Shah, MBBS, DCH, DNB, DM817/B, Padmini Darshan, Jayanagar 7th block; Bangalore 560080, KarnatakaIndiaEmail: krunalshah14@yahoo.comPublication History
Article published online:
20 October 2022© 2022. Indian Society of Medical and Paediatric Oncology. This is an open access article published by Thieme under the terms of the Creative Commons Attribution-NonDerivative-NonCommercial License, permitting copying and reproduction so long as the original work is given appropriate credit. Contents may not be used for commercial purposes, or adapted, remixed, transformed or built upon. (https://creativecommons.org/licenses/by-nc-nd/4.0/)
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India

| Figure 1:Pneumothorax with tracheal shift to right
References
- Groll AH, Pana D, Lanternier F. et al; 8th European Conference on Infections in Leukaemia. 8th European Conference on Infections in Leukaemia: 2020 guidelines for the diagnosis, prevention, and treatment of invasive fungal diseases in paediatric patients with cancer or post-haematopoietic cell transplantation. Lancet Oncol 2021; 22 (06) e254-e269
- Pappas PG, Alexander BD, Andes DR. et al. Invasive fungal infections among organ transplant recipients: results of the Transplant-Associated Infection Surveillance Network (TRANSNET). Clin Infect Dis 2010; 50 (08) 1101-1111
- Abbasi S, Shenep JL, Hughes WT, Flynn PM. Aspergillosis in children with cancer: A 34-year experience. Clin Infect Dis 1999; 29 (05) 1210-1219
- Das N, Banavali S, Bakhshi S. et al. Protocol for ICiCLe-ALL-14 (InPOG-ALL-15-01): a prospective, risk stratified, randomised, multicentre, open label, controlled therapeutic trial for newly diagnosed childhood acute lymphoblastic leukaemia in India. Trials 2022; 23 (01) 102
- Pana ZD, Roilides E, Warris A, Groll AH, Zaoutis T. Epidemiology of invasive fungal disease in children. J Pediatric Infect Dis Soc 2017; 6 (suppl_1): S3-S11
- Tragiannidis A, Kattamis A, Vyzantiadis TA. Invasive fungal infections in children with haematological malignancies: diagnostic and therapeutic challenges. J Fungi (Basel) 2021; 7 (07) 516
- Donnelly JP, Chen SC, Kauffman CA. et al. Revision and update of the consensus definitions of invasive fungal disease from the European Organization for research and Treatment of cancer and the Mycoses Study Group education and research consortium. Clin Infect Dis 2020; 71 (06) 1367-1376
- Grigull L, Beier R, Schrauder A. et al. Invasive fungal infections are responsible for one-fifth of the infectious deaths in children with ALL. Mycoses 2003; 46 (11,12): 441-446
- Denning DW, Marinus A, Cohen J. et al; EORTC Invasive Fungal Infections Cooperative Group. An EORTC multicentre prospective survey of invasive aspergillosis in haematological patients: diagnosis and therapeutic outcome. J Infect 1998; 37 (02) 173-180
- Cornillet A, Camus C, Nimubona S. et al. Comparison of epidemiological, clinical, and biological features of invasive aspergillosis in neutropenic and nonneutropenic patients: a 6-year survey. Clin Infect Dis 2006; 43 (05) 577-584
- Thomas KE, Owens CM, Veys PA, Novelli V, Costoli V. The radiological spectrum of invasive aspergillosis in children: a 10-year review. Pediatr Radiol 2003; 33 (07) 453-460
- Burgos A, Zaoutis TE, Dvorak CC. et al. Pediatric invasive aspergillosis: a multicenter retrospective analysis of 139 contemporary cases. Pediatrics 2008; 121 (05) e1286-e1294
- Ödev K, Çaliskan U, Emlik D, Koç H, Koç S. Pneumomediastinum and pneumopericardium due to intracavitary aspergilloma: an unusual complication of fungal pneumonia. Pediatr Radiol 2002; 32 (02) 143-145
- Todeschini G, Murari C, Bonesi R. et al. Invasive aspergillosis in neutropenic patients: rapid neutrophil recovery is a risk factor for severe pulmonary complications. Eur J Clin Invest 1999; 29 (05) 453-457
- Huppler AR, Fisher BT, Lehrnbecher T, Walsh TJ, Steinbach WJ. Role of molecular biomarkers in the diagnosis of invasive fungal diseases in children. J Pediatric Infect Dis Soc 2017; 6 (suppl_1): S32-S44


 PDF
PDF  Views
Views  Share
Share

