Quantification of telomerase activity in normal oral mucosal tissue and oral squamous cell carcinoma
CC BY-NC-ND 4.0 · Indian J Med Paediatr Oncol 2016; 37(03): 183-188
DOI: DOI: 10.4103/0971-5851.190350
Abstract
Background and Objective: The role of telomeres and telomerase in oral cancer is an area of much recent interest. The understanding of the role of telomere biology, the end replication problem leading to genomic instability and the reactivation of telomerase, is absolutely critical to our understanding of oral cancer, and more so, to our ability of early diagnosis and developing novel therapies and cancer prevention approaches. The aim of the present study was to quantify telomerase activity (TA) in oral squamous cell carcinoma (OSCC) and normal oral mucosa and assess the role of telomerase as diagnostic and prognostic marker of oral malignancy. Materials and Methods: We quantified TA in 45 patients with OSCC and 20 normal oral mucosal specimens using polymerase chain reaction-based telomeric repeat amplification protocol assay and compared it with the clinical status and grade of malignancy. Results: TA was detected in 89% of malignant and 5% of normal oral mucosal tissue. The TA levels ranged from 0.28 to 6.91 (mean 2.05, standard deviation [SD] 1.33) in OSCC and 0.21 to 1.09 (mean 0.54, SD 0.27) in normal oral mucosa. There was no relationship between TA levels and clinical stages, site of the lesion, history of adverse habits, or sex of the patient. However, under the WHO classification, there were significant differences P < 0.00) between Grades I, II, and III. Furthermore, increasing age of the patient significantly correlated with TA. Interpretation and Conclusion: The results of the present study indicate that activation of TA is frequent in OSCC. Statistically significant difference in quantified telomerase levels of OSCC and normal oral mucosa P < 0.00) demonstrates the significant clinical usefulness of telomerase activation as a valuable marker for diagnosis while significant correlation of TA with grades of malignancy indicates its effectiveness as marker for prognosis of OSCC.
Publication History
Article published online:
12 July 2021
© 2016. Indian Society of Medical and Paediatric Oncology. This is an open access article published by Thieme under the terms of the Creative Commons Attribution-NonDerivative-NonCommercial-License, permitting copying and reproduction so long as the original work is given appropriate credit. Contents may not be used for commercial purposes, or adapted, remixed, transformed or built upon. (https://creativecommons.org/licenses/by-nc-nd/4.0/.)
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India
Abstract
Background and Objective:
The role of telomeres and telomerase in oral cancer is an area of much recent interest. The understanding of the role of telomere biology, the end replication problem leading to genomic instability and the reactivation of telomerase, is absolutely critical to our understanding of oral cancer, and more so, to our ability of early diagnosis and developing novel therapies and cancer prevention approaches. The aim of the present study was to quantify telomerase activity (TA) in oral squamous cell carcinoma (OSCC) and normal oral mucosa and assess the role of telomerase as diagnostic and prognostic marker of oral malignancy.
Materials and Methods:
We quantified TA in 45 patients with OSCC and 20 normal oral mucosal specimens using polymerase chain reaction-based telomeric repeat amplification protocol assay and compared it with the clinical status and grade of malignancy.
Results:
TA was detected in 89% of malignant and 5% of normal oral mucosal tissue. The TA levels ranged from 0.28 to 6.91 (mean 2.05, standard deviation [SD] 1.33) in OSCC and 0.21 to 1.09 (mean 0.54, SD 0.27) in normal oral mucosa. There was no relationship between TA levels and clinical stages, site of the lesion, history of adverse habits, or sex of the patient. However, under the WHO classification, there were significant differences (P < 0.00) between Grades I, II, and III. Furthermore, increasing age of the patient significantly correlated with TA.
Interpretation and Conclusion:
The results of the present study indicate that activation of TA is frequent in OSCC. Statistically significant difference in quantified telomerase levels of OSCC and normal oral mucosa (P < 0.00) demonstrates the significant clinical usefulness of telomerase activation as a valuable marker for diagnosis while significant correlation of TA with grades of malignancy indicates its effectiveness as marker for prognosis of OSCC.
INTRODUCTION
Oral cancer is significant component of the global burden of cancer affecting more than 400,000 individuals per year worldwide.[1] It is the most common neoplasm in India and most Southeast Asian countries, the high incidence of which has been attributed to the prevalence of tobacco chewing among the populations of these countries.[2] Based on the increasing incidence of head and neck cancers, problems associated with late diagnosis, and the public health dilemma they represent, it would seem prudent to enact screening protocols to check at-risk people. Early diagnosis would allow for conservative therapeutic approaches with a brief recovery and a more favorable prognosis.
One aspect of cancer research that has intrigued most scientists is the acquisition of unlimited proliferative potential of cancer cells. In the process of transformation of a cell from normal state to malignant, one of the key steps is the immortalization of cells. Recent evidence indicates that telomerase plays an important role in cell immortalization. Telomerase is an RNA-dependent DNA polymerase that synthesizes telomeric DNA fragments de novo, using its RNA moiety as a template, and compensates for the loss of telomere during the cell division.[3] Telomeres are specialized heterochromatic structures at the ends of vertebrate chromosomes that have been implicated in stabilizing and protecting the chromosomes, anchoring chromosomes within the nucleus, and assisting the replication of linear DNA.[4] Approximately 50–200 bp of telomeric DNA in somatic cells is lost on each population doubling, which is implicated in the control of the life span.[5] Telomerase is active in embryonal and germ line cells but remains silent in most differentiated somatic cells.[6] Loss of telomerase activity (TA) has been correlated with cellular senescence whereas its reactivation may be prerequisite for the development of malignant tumor cells from somatic cells.[7] More than 85% of human cancer cells are found to have TA.[6] Oral squamous cell carcinoma (OSCC) shows highest TA while oral potentially malignant lesions have relatively lower TA.[8] Hence, TA is considered to be a diagnostic marker of malignancy. In addition, telomerase has been found to be upregulated according to tumor progression and may, therefore, serve as a useful prognostic indicator in identifying the patients in need of a close follow-up and vigorous adjuvant treatment.[9] Moreover, telomerase is an important target for the design of therapeutic agents that might have minimal side effects.[10]
Most of the previous studies on TA in OSCC have qualitatively assessed TA positivity rates or semiquantitatively evaluated relative TA. The present study aims at quantification of TA in OSCC and normal oral mucosal tissue and comparison of the same. In addition, correlation of TA with clinical disease status and pathologic features such as tumor differentiation is investigated to evaluate the clinical significance and validity of TA as useful biomarker not only for early detection of cancer but also in prognosis of OSCC.
MATERIALS AND METHODS
The study group comprised 45 histopathologically proven cases of OSCC and the control group comprised 20 normal oral mucosal samples obtained from healthy individuals who underwent surgical extraction of impacted third molars. Informed written consent was obtained from all patients. The study subjects were selected randomly. Patients who had previously undergone or were under any form of therapy for OSCC, patients suffering from recurrence of OSCC, and patients on long-term NSAIDs or corticosteroid therapy (known to inhibit TA) were excluded from the study. Demographic data and clinical details of each patient were also recorded using a structured questionnaire.
Sixty-five fresh tissue specimens obtained from oral cavity were immediately placed in RNA stabilizing reagent and frozen for cell extract preparation. The study protocol was divided into three major steps – RNA extraction, telomeric repeat amplification protocol (TRAP) assay, and Gel electrophoresis.
RNA extraction
The tissue specimens placed in RNA stabilizing reagent and frozen were washed twice in cold phosphate-buffered saline (PBS) by adding 500 μl PBS to the sample placed in microfuge tube and centrifuging at 5000 ×g for 1 min. The tissue specimens were then treated with 1 ml Lysis Buffer and incubated on ice for 30 min. Cell debris was pelleted (12,000 ×g for 30 min at 4°C), and the supernatant was collected and frozen at −80°C for future use. Protein concentration of the tissue was determined using biophotometer (Eppendorf, USA). Aliquots of extracts containing 20 μg protein were used for each TRAP assay.
Telomeric repeat amplification protocol assay
TRAP assay for test reactions (OSCC and normal oral mucosa samples) consisted of two steps. The aliquoted supernatant or cell extract was thawed to room temperature. A volume of 2.5 μl of cell extract was mixed with 1 μl of CX reverse primer and 6.5 μl of diethylpyrocarbonate (DEPC) to make the volume of the RNA primer mix to 10 μl. The above mix was incubated at 65°C for 10 min and immediately chilled on ice. This step allowed telomerase in the extract to synthesize telomeric oligonucleotides on TS primer.
Master mix was prepared by adding the components in the following order: 200 μl of reaction mix provided in the kit was added, followed by 16 μl each of TS forward primer and enzyme mix, respectively. Finally, 16 μl of DEPC water was added to complete the master mix composition. 15.5 μl of this master mix was added in each polymerase chain reaction (PCR) tube having 10 μl of RNA primer mix. In the second step, the telomerase-synthesized new oligonucleotides were amplified using PCR by including reverse CX primer in the presence of deoxynucleotide triphosphates.
The above-prepared final mix was placed in a thermocycler in which three-step PCR was performed. The thermal cycler was preheated to 50°C before placing samples in it. The cycle conditions were 42°C for 30 min followed by 94°C for 15 min for denaturation process. The annealing step comprised 36 cycles of 94°C for 30 s, 58°C for 30 s, and 72°C for 30 s. Following the annealing step, extension was done at 72°C for 5 min and 4°C for 1 min. This final extension completed the unfinished ends. The products of reaction were removed from thermocycler and stored at 4°C for gel electrophoresis.
Control reaction which was later used as internal standard for comparison of TA was repeated at several dilutions (1:2, 1:5, 1:10, 1:25, and 1:50) of control RNA template.
Gel electrophoresis
After 7 μl of bromophenol blue dye was added to each tube containing the PCR product, the products of the reaction (10 μl of each) were resolved by electrophoresis in a 12% nondenaturing polyacrylamide gel containing ethidium bromide, in 10X TAE (Tris acetate ethylenediaminetetraacetic acid). Ten microliters of control reaction PCR products in the above-mentioned several dilutions were also resolved on the gel. The minimum dilution of control reaction at which detectable activity could be appreciated was 1 μg. Therefore, for each run of test reactions, a control reaction PCR product (1 μg) was included for later comparison. The gel was run for 2 h at 25 V and 16 mA. After separation of products in ladder pattern, the gel was removed and wet gel was scanned directly using 254 nm ultraviolet transilluminator [Figure 1]. Ethidium bromide that had bound specifically to nucleic acids depending on the molecular weight and concentration of the nucleic acid was excited by ultraviolet irradiation, and on excitation, it emitted fluorescent light which was captured by built-in camera.
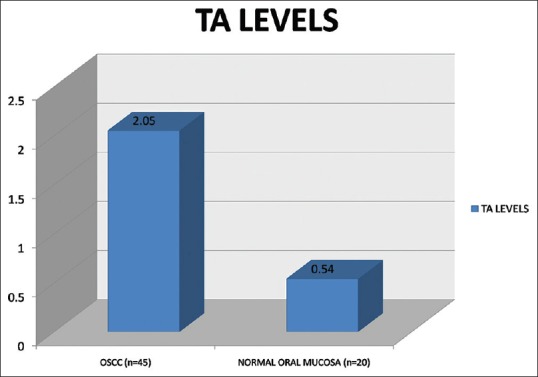
| Fig. 1 The bar diagram depicts the mean quantified telomerase activity in oral squamous cell carcinoma and normal mucosa
Data analysis
Total Lab Phoretic Software (Nonlinear Dynamics Limited, UK) was used for gel analysis. The sum of total integrated fluorescence intensities of telomerase ladders in each gel lane from test reaction was divided by the signal from the co-amplified internal standard (control RNA reaction) to get quantified telomerase levels. Since the TA of control RNA template was 1 μg, this was considered as the cutoff value. The test reactions showing TA levels of > 1 were considered positive and those showing TA levels of < 1 were considered negative.
Statistical analysis
TA levels were compared between OSCC and normal oral mucosa using Student's t-test. The relationship between the level of TA and clinicopathologic factors such as age, sex, adverse habits, intraoral site of the lesion, size of the lesion, lymph node status, stage of the disease, and histopathologic grade was analyzed. Student's t-test and ANOVA test were applied to validate the significance of the difference between groups. Pairwise comparison among the WHO grades of malignancy and TA levels was done by Tukey's post hoc procedure.
RESULTS
The results of the present study showed TA in 89% of OSCC and 5% of normal oral mucosa. The TA levels ranged from 0.28 to 6.91 (mean 2.05, standard deviation [SD] 1.33) in OSCC and 0.21 to 1.09 (mean 0.54, SD 0.27) in normal oral mucosa. TA level differed significantly between OSCC and normal oral mucosa (t = 4.9691, P = 0.0000) [Figure 1].
On comparison of TA activity with clinical and pathological parameters, it was found that there was a statistically significant difference in TA levels between patients above 50 years and above and below 50 years of age (P = 0.0298). The Student's t-test did not show statistically significant difference in the mean TA levels among males and females, among patients with or without adverse habits, and also did not differ among groups with different types of tobacco habits. Significant difference in TA levels at different primary site of OSCC was not observed such as tongue and buccal mucosa. TA levels among T1 and T2 were found to be higher than T3. TA levels increased slightly but not significantly with progression from T3 to T4a and T4b. The TA levels in T3 + T4 disease were slightly but not significantly higher than that in T1 + T2 disease (P = 0.1060). TA levels increased with progression of N disease. However, TA levels in N2a were found to be lower than N1. The TA levels of N2b and N2c nodal disease were almost equal. TA levels in patients with regional lymph node metastasis (N1 + N2) were slightly but not significantly higher than N0 disease (P = 0.3623). A statistically significant difference in TA according to overall clinical stage was not observed in the present study (P = 0.2703). The TA levels did not show pattern of increase with progression of clinical stage. Furthermore, statistically significant differences between early stages (I + II) and late stages (III + IV) were not observed in the present study with the P value being 0.8621 [Table 1].
Table 1
ANOVA test for comparison of telomerase activity levels among clinical stages oral squamous cell carcinoma
 A higher histological grade of malignancy in WHO classification was associated with a higher TA level [Table 2] and the difference was statistically significant (P = 0.0000).
A higher histological grade of malignancy in WHO classification was associated with a higher TA level [Table 2] and the difference was statistically significant (P = 0.0000).Table 2
ANOVA test for comparison of telomerase activity levels among WHO classification of grades of oral squamous cell carcinoma

DISCUSSION
The telomere hypothesis of cancer cell immortalization remains an attractive concept and TA in human oral cancer is a subject of great interest. The highly sensitive PCR-based TRAP assay provides the means to analyze TA in a wide variety of tissues. This method makes it possible to detect TA in a very small number of cells which is a major advantage for examining clinical specimens. The overall prevalence of 85% among more than 3000 human tumor samples tested using TRAP assay makes the TA the most universal marker for human cancers.[11] The diagnostic and prognostic potential of telomerase could contribute to improving the outcome of patients with OSCC. Although the incidence of OSCC is so high, TA in this tumor has not been extensively studied. There are several reports from all over the world and only a few from India, but the data remain limited. In addition, most of the previous literature is limited to qualitative or semiquantitative estimation of TA. In order for cells to gain the ability to proliferate excessively and become immortal, a certain level of TA is needed.[12]
The present study was aimed at quantitative detection of TA in normal oral mucosal tissue and OSCC. We investigated the clinical significance of telomerase activation in head and neck cancer patients in India. TA was detected in 89% of OSCC samples as compared to 5% of normal oral mucosal samples. This is in accordance with previous studies in which TA detected in head and neck squamous cell carcinoma (HNSCC) has ranged from 75% to 100% of the samples investigated. Califano et al.,[13] Liao et al.[14] and Koscielny et al.[15] in their studies have demonstrated TA of < 85% in HNSCC. Higher TA values have been reported in OSCC by Mao et al.,[16] Curran et al.,[17] Sumida et al.,[18] Miyoshi et al.,[19] and Fujita et al.[8] Thurnher et al.[20] detected TA in 100% of OSCC samples in their study. Among the three studies conducted in the Indian population, Kannan et al.[2] and Patel et al.[9,21] have reported TA of 75%, 80%, and 72.8% in OSCC, respectively. These results suggest that telomerase is activated in OSCC and this is in keeping with the concept that the enzyme plays a key role of telomere maintenance in carcinogenesis. However, it was also noted that the acquisition of TA was not an obligate pathway for oral carcinogenesis since 11% OSCC lacked this activity. The telomerase negative samples may exhibit alternative lengthening mechanism of telomere maintenance in form of chromosome maintenance or other uncharacterized mechanisms. We detected weak TA in 1/20 (5%) of normal oral mucosal samples. Previous literature on this subject has shown conflicting results. While Mao et al.,[16] Sumida et al.,[18] Chang et al.,[22] and Miyoshi et al.[19] have not reported any detectable TA in normal tissues, Liao et al.,[14] Xian et al.,[23] and Fujita et al.[8] have made a distinctive observation of the presence of weak TA in normal samples. The telomerase positivity rates in these studies have ranged from 2.78% to 100% for normal samples. The reason for telomerase positivity in one of the normal oral mucosal samples in the present study may be related to stratification and complex differentiation pattern of squamous epithelium. Squamous epithelium has very high cell turnover rate, and the constant loss of superficial cells by the process of desquamation is replaced by continuous division in the basal cells. Hence, comparatively more stem cells are likely to be present in the squamous epithelium for the active self-renewal process.[24] As in the case of immortalized cells, the stem cells of human hematopoietic tissue have been shown to possess TA.[25] Cellular kinetic studies have also revealed a similarity in cell proliferation pattern between the stem cells of squamous epithelium and hematopoietic tissues.[26] Thus, the TA in normal oral mucosa may be due to the presence of stem cells. However, Bickenbach et al.[27] have reported that epidermal stem cells were not the major source of TA and demonstrated that proliferating transit-amplifying cells exhibited more active telomerase. It has also been demonstrated that a normal hematopoietic cell lineage has weak TA and that the telomere shortening occurred in the hematopoietic cell lineage which is subject to aging, implying that the level of TA in those cells may be insufficient for the prevention of telomere erosion.[14] A similar process may be present in oral epithelium which might be responsible for weak TA.
In the present study, quantification of TA in OSCC and normal oral mucosal samples showed a statistically significant difference between TA of OSCC (2.05) and normal oral mucosa (0.54). These results are in accordance with previous studies by Fujita et al.[8] and Yajima et al.[12] Our preliminary results indicate that TA may be a valuable marker for diagnosis of OSCC.
In this study, TA was broadly distributed among OSCC samples ranging from 0.28 to 6.91. The wide distribution of TA is attributed to the mechanism of immortalization involving factors other than telomerase activation. In addition, possibility of mesenchymal tissue contamination, lymphoid proliferation, and necrotic tissue contamination has to be considered. Sumida et al.[18] considered the possibility of contamination by mesenchymal tissue and necrotic lesions which might affect telomere extension and PCR and thereby influence results of TA assay. TA of normal lymphocytes may also confound the interpretation of the results. Since TA was reported in normal hematopoietic cells, we also looked for any differences in inflammatory cell infiltration in telomerase-negative and telomerase-positive lesions with routine histopathological examination. No significant difference between these groups with regard to inflammatory cell infiltration was observed because the telomerase-negative lesions also had marked inflammatory cell infiltration, similar to telomerase-positive lesions. Telomerase is a highly stable ribonucleoprotein complex, half-life of which is over 20 h in vivo.[9] Therefore, samples obtained by biopsy or during surgery or autopsy can be analyzed by the TRAP assay. However, in case of oral lesions, contamination by bacteria, plaque material, saliva, and blood may complicate the results.
In our study, we analyzed TA in tissue sections immediately adjacent to sections for which histological features were reviewed by bisecting the clinical specimen into two parts. Using this technique, we were able to compare TA in tumors and adjacent tissues with their precise histological features. The degree of TA in OSCC was related to the histological differentiation. A higher histological grade of malignancy in WHO classification was associated with a higher TA level. It has been reported that WHO Grade I OSCC are slow-growing, compared with Grade II and III OSCC, which are also more aggressive in nature. It is likely that less differentiated OSCC (Grades II and III) might contain more immortal cells than the more differentiated histological type (Grade I); hence, TA would also be high.
The presence as well as the absence of a relation between TA and the WHO classification has been reported previously. A semiquantitative analysis performed by Kannan et al.[2] revealed high TA levels in 10 (29.4%) of 34 well-differentiated OSCC lesions and 7 (100%) of 7 moderately to poorly differentiated OSCC lesions, suggesting that a lower histological grade of differentiation is associated with higher TA. The results of semiquantitative assay by Sumida et al.[18] indicated that Grade III OSCC possessed higher TA than Grade I OSCC. However, Miyoshi et al.[19] reported the absence of such a relationship. However, they analyzed 19 Grade I lesions but only 1 Grade II and 1 Grade III lesion; therefore, their results may not support the absence of a relation between TA and tumor grade. Patel et al.[9,21] also failed to attain any correlation with grades of malignancy. Fujita et al.[8] found that relative TA varied widely among Grades II and III lesions, with no significant difference. However, most Grade I lesions had low relative TA and TA level differed significantly between Grades I and III and between Grades I and II + III, in their study. The available data indicate that telomerase may be associated with increased cancer cell atypia and mitotic figures and structural changes of cancer tissue.
CONCLUSION
Telomerase reactivation is a necessary and rate-limiting step of cellular immortalization and could provide a unique marker of aberrant cells, which may selectively be targeted.[28]
Although telomerase was initially considered a specific marker of malignancy, weak activity of normal tissue can lead to false-positive results. A quantitative telomerase assay that can distinguish between normal tissue and malignancy is, therefore, required. Because of this expression pattern, testing for TA may deliver useful diagnostic information about clinical tumor behavior. It can be concluded from the current study that enhanced expression of TA occurs during human oral carcinogenesis and supports the critical role of telomerase in the development of human oral cancer.
TA levels showed no correlation with clinical parameters such as sex of the patient, history of adverse habits, site of the lesion, size of the lesion, lymph node involvement, or overall stage of the patient. However, significant correlation with age of the patient and histopathological grades of malignancy was observed in the present study. Thus, TA can be an additional tool, as a biomarker, for assessing the prognosis or biological grade of the oral carcinomas.
Financial support and sponsorship
Nil.
Conflicts of interest

| Fig. 1 The bar diagram depicts the mean quantified telomerase activity in oral squamous cell carcinoma and normal mucosa


 PDF
PDF  Views
Views  Share
Share

