Radiation Recall Dermatitis in Breast Cancer Patient after Trastuzumab: A Case Report with Review of Literature
CC BY 4.0 · Indian J Med Paediatr Oncol 2023; 44(03): 365-370
DOI: DOI: 10.1055/s-0043-1761263
Abstract
Radiation recall dermatitis (RRD) is an extremely rare phenomenon. A variety of factors such as antineoplastic agents, pharmaceutical agents, physical and environmental factors have been proposed to be the underlying cause of RRD. Only a handful cases have been reported till date, where trastuzumab is sought to be the triggering agent. The presentation of RRD varies from mild erythematous to extensive confluent dermatitis, resolving over a period of 1 to 2 weeks with conservative management. Most of the patients tend to tolerate rechallenge well without showing reappearance. We hereby describe a lady with breast cancer having RRD following administration of trastuzumab. She developed reaction 28 days post-radiotherapy and managed conservatively. Furthermore, she was rechallenged with the same dose, that she tolerated very well, without any reappearance. Hence, an acquaintance of the clinicians to this rare entity is essential for timely diagnosis and appropriate management.
Publication History
Article published online:
12 May 2023
© 2023. The Author(s). This is an open access article published by Thieme under the terms of the Creative Commons Attribution License, permitting unrestricted use, distribution, and reproduction so long as the original work is properly cited. (https://creativecommons.org/licenses/by/4.0/)
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India
Abstract
Radiation recall dermatitis (RRD) is an extremely rare phenomenon. A variety of factors such as antineoplastic agents, pharmaceutical agents, physical and environmental factors have been proposed to be the underlying cause of RRD. Only a handful cases have been reported till date, where trastuzumab is sought to be the triggering agent. The presentation of RRD varies from mild erythematous to extensive confluent dermatitis, resolving over a period of 1 to 2 weeks with conservative management. Most of the patients tend to tolerate rechallenge well without showing reappearance. We hereby describe a lady with breast cancer having RRD following administration of trastuzumab. She developed reaction 28 days post-radiotherapy and managed conservatively. Furthermore, she was rechallenged with the same dose, that she tolerated very well, without any reappearance. Hence, an acquaintance of the clinicians to this rare entity is essential for timely diagnosis and appropriate management.
Keywords
radiation recall dermatitis - trastuzumab - radiation recall phenomenonIntroduction
Radiation recall is an ill-defined inflammatory phenomenon characterized by reactions triggered by exposure to a certain agent in the previously irradiated region.[1] It is triggered by post-radiation exposure to certain offending agents including antineoplastic and other pharmacological agents, physical and environmental factors.[1] [2] [3] Radiation recall dermatitis (RRD) is the most common manifestation of radiation recall phenomenon.[3] The first documented evidence of RRD was reported long back in 1959 by D'Angio et al.[4] Presently more than hundred cases have been reported in the form of either isolated case reports or small case series. The estimated incidence of RRD is around 6 to 8%.[1] [2] [3]
We report a case of RRD in breast cancer patient triggered by trastuzumab along with a review of literature of similar cases. A literature review was done for all published case reports or case series in English language on RRD with trastuzumab using the keywords “radiation recall dermatitis,” “trastuzumab,” and “radiation recall phenomenon.”
Case Report
A 59-year-old postmenopausal hypertensive lady without any significant family history or any history of allergy evaluated for a 5 × 4 cm lump in the left breast and a 1 × 1 mobile axillary lymph node in June 2021. Histopathology confirmed it as invasive breast carcinoma, no special type, grade 3, hormone receptor positive (estrogen receptor: Allred score—8, progesterone receptor: Allred score— 7) and Her 2 Neu positive on immuno-histochemistry. Staging 18F-fluorodeoxyglucose positron emission tomography/computed tomography scan depicted a soft tissue lesion of 49 × 42 mm in upper inner quadrant with a small satellite nodule in lower outer quadrant along with axillary lymph nodes without any distant metastases. She received three cycles of multiagent neoadjuvant chemotherapy consisting TCH regimen (docetaxel 75 mg/m2, carboplatin area under the curve 6, and trastuzumab loading dose of 6 mg/kg followed by 4 mg/kg) that led to partial clinicoradiological partial response. She underwent modified radical mastectomy 4 weeks after completion of chemotherapy. The final histopathology report revealed a unifocal tumor of maximum size of 2 cm with 1 out of 38 dissected lymph nodes was positive without extranodal extension (stage- ypT1c ypN1a). Later, she received adjuvant chemotherapy with three more cycles of TCH. Further, she was started on three weekly maintenance trastuzumab along with anastrozole.
Four weeks post-adjuvant TCH and one week after seventh cycle of trastuzumab, she received locoregional radiotherapy (LRRT) targeting left chest wall (CW) and left supraclavicle fossa (SCF). LRRT was delivered using 6 MV photons to a total dose of 40 Gy in 15 fractions over a period of 3 weeks via bitangential portals for CW and a single anterior portal for SCF radiation. The entire treatment was performed by deep inspiratory breath hold technique and a 5 mm thick bolus was placed throughout the course of radiation over the CW for adequate coverage of the skin. Maximum dose (D max) to the planning target volume (PTV) was 107.2%-and volume receiving 105%-(V105%) was 11.6 cc; all the other dosimetric parameters for PTV and organs at risk were within the predefined limits.[5] She tolerated LRRT well and at the end of LRRT, she had radiation therapy oncology group (RTOG) grade 1 dermatitis and grade 1 esophagitis at the completion of radiation that were well managed with topical steroid creams and anesthetic antacid gel. In the last week of LRRT, she received her eighth cycle of trastuzumab without any undue toxicity. After 1 week of completion of LRRT, she presented with focal moist desquamation along the scar over the CW ([Fig. 1A]) for which she was prescribed placental extract gel. Two weeks later, ninth cycle of trastuzumab was given (14 days post-LRRT).
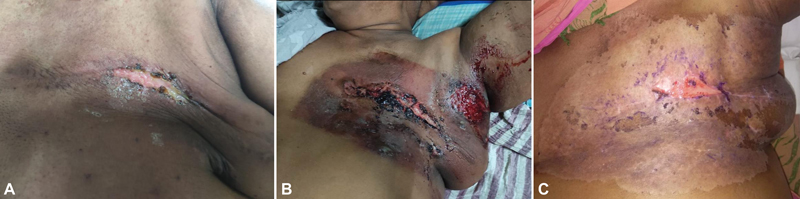
| Fig 1 :(A) Area of moist desquamation, 7 days post-LRRT. (B) Radiation recall dermatitis (RRD), 14 days post-trastuzumab, 28 days post-LRRT. Reaction was well demarcating radiation chest wall portals. (C) Near complete resolution of RRD with small persistent area of moist desquamation along the scar, 42 days post-LRRT. LRRT, locoregional radiotherapy;
In the subsequent week, she had progressive worsening of dermatitis and after 2 weeks (28 days post-LRRT), she landed up with worsening RTOG grade 3 dermatitis. Intense dermatitis in the form of ulceration, small areas of hemorrhage, was noted over the entire CW ([Fig. 1B]). However, the reaction was restricted within the LRRT portals and no reaction was observed outside the irradiated region, leading to the diagnosis of RRD. She was managed with topical 1% gentian violet (GV) application along with analgesics. There were no signs or evidence or any superadded infection. Surprisingly, no reaction was observed over the site of SCF irradiation. High-resolution computed tomography chest ruled out underlying recall pneumonitis. Gradually over a period of 3 weeks (42 days post-LRRT), the reaction showed significant improvement with near complete resolution with a persistent small area of moist desquamation along the scar that healed completely in next 2 weeks ([Fig. 1C]). After 40 days from ninth cycle (54 days post-LRRT), she was rechallenged with the same dose of trastuzumab, without any reappearance of recall reaction.
Discussion
RRD is a well-known entity but largely under-reported.[1] Most of the reported cases are with chemotherapy agents,[2] [6] [7] followed by some non-neoplastic agents,[8] [9] physical agents,[3] [10] and other pharmaceutics.[11] [12] However, only a few case reports highlight this reaction following targeted therapies[13] including trastuzumab.[13] [14] [15] [16] [17] [18] [19] The overexpression of the HER2 is observed in 20 to 30% of primary breast cancers[20] and trastuzumab is a recombinant humanized immunoglobulin G1 monoclonal antibody against HER2, indicated for the management of both primary breast cancer and metastatic disease.[20] The most serious and/or common adverse reactions reported with trastuzumab usage are cardiac dysfunction, infusion-related reactions, neutropenia, and pulmonary adverse reactions.[20] Although dermatitis with severity ranging from mild-to-moderate has been reported with the use trastuzumab,[20] [21] radiation recall is extremely rare and all documented cases have developed reaction to the irradiated skin (RRD),[13] [14] [15] [16] [17] [18] [19] with only a single reported case of radiation recall pneumonitis[17] till date.
All cases depicting RRD triggered by trastuzumab[13] [14] [15] [16] [17] [18] [19] are summarized in [Table 1]. Average duration between radiotherapy (RT) and occurrence of RRD was noted to be 135 days (range: 29–283 days). The triggering cycle of trastuzumab for development of RRD and the cumulative doses at the occurrence of RRD are highly variable in the literature. Moreover, the development of RRD does not seem to be related to RT tolerance as most of the patients developed RRD despite a good tolerance. The RT dose fractionation and target volumes also do not seem to have any corelation with the incidence or intensity of RRD, as majority of these cases are reported with hypofractionated LRRT. However, Alsabbak et al have observed the reaction all over the treated region of breast but with an increased intensity over the area of RT boost region.[14]
|
Sr. No. |
Author |
Patient characteristics |
Radiotherapy details |
Triggering agent |
Description of RRD |
Treatment and outcome |
Rechallenge |
|---|---|---|---|---|---|---|---|
|
1. |
Shrimali et al, 2009[16] |
A 71-year-old female with breast cancer, history of allergies: NR |
45Gy in 20# to CW and SCF, at conclusion she had erythematous dermatitis |
Trastuzumab (dose: NS) every 3 weeks with anastrozole (1mg/day), started 42 days after RT |
Mild, asymptomatic erythematous RRD noticed 3 weeks after first cycle (62 days after RT) |
Intravenous hydrocortisone and oral paracetamol. Complete resolution of RRD (duration: NS) |
Yes, under steroid coverage with same dose, without reappearance |
|
2. |
Chung et al, 2009[18] |
A 41-year-old female with breast cancer, history of eczema, allergic rhinitis, and contact dermatitis to numerous allergens |
42.5Gy in 16# to WB and 10 Gy TBB and 37.5Gy in 16# to SCF, IMN, and axilla. Post-RT she had brisk erythema and moist desquamation over inframammary fold |
Trastuzumab (513.28 mg) IV every 3 weeks, started 28 days after RT |
Mild, painful, swollen, erythematous RRD, 3 days after 12th cycle (283 days post-RT) |
Nil. Brisk erythema resolved spontaneously within 2 days, pain persisted for ∼14 days |
Yes, without reappearance |
|
3. |
Moon et al, 2013[15] |
A 55-year-old female with breast cancer, no past history of allergies |
45Gy in 25# to WB, SCF and IMN, axilla with TBB 9Gy in 5#. At conclusion she had erythematous dermatitis |
Trastuzumab (6mg/kg) every 3 weeks, 45 days after RT |
Mild, erythematous RRD, noticed 9 days after fifth cycle (159 days post-RT) |
Nil. Resolved completely in 7 days |
Yes, same dose, without reappearance |
|
4. |
Alsabbak et al, 2013[14] |
A 47-year-old female with breast cancer, history of allergies: NR |
50 Gy in25# to CW and 14Gy in 7# boost to area of positive margins by 9 MeV electrons with 1 cm bolus. At conclusion she had erythematous dermatitis with a small area of desquamation |
Trastuzumab (dose: NS) every 3 weeks, continued during RT |
Mild, erythematous RRD 2 weeks after third cycle (56 days post-RT). RRD was most prominent in the area of boost |
Benadryl and topical steroid. Complete resolution of RRD (duration: NS) |
Yes, after 3 weeks. No reappearance |
|
5. |
Levy et al, 2013[13] |
Age: NS, female with breast cancer, history of allergy: NR |
50Gy in 25#, Site: NS |
Trastuzumab (dose: NS), started 25 weeks after RT |
Severity of RRD: NS, developed in 2 weeks after exposure (189 days post-RT) |
NS |
NS |
|
6. |
Lee et al, 2014[17] |
A 55-year-old female with fibroadenoma of breast with axillary metastases, history of allergy: NR |
50.4Gy in 20# to WB |
Trastuzumab (dose: NS) every 3 weeks, started 10 days after RT |
Mild, erythematous RRD and edematous plaques, developed 24 weeks after RT (168 days post-RT) along with radiation recall pneumonitis |
Prednisolone, 30mg, Improvement in 2 weeks |
NS |
|
7. |
Anupama et al 2018[19] |
A 56-year-old female with breast cancer, past history of allergies: No |
40 Gy/15# to CW and SCF, at conclusion: mild erythematous dermatitis |
Trastuzumab (450mg) every 4 weeks, started 4 weeks after RT |
Mild-to-moderate, painful, swollen and erythematous, maculopapular RRD with discoloration, next day of first cycle (29 days post-RT) |
Topical betamethasone cream, erythema reduced in 2 days but pain persisted for 2 weeks |
Yes, after 4 weeks, no reappearance |
References
- Azria D, Magné N, Zouhair A. et al. Radiation recall: a well recognized but neglected phenomenon. Cancer Treat Rev 2005; 31 (07) 555-570
- Burris III HA, Hurtig J. Radiation recall with anticancer agents. Oncologist 2010; 15 (11) 1227-1237
- Camidge R, Price A. Characterizing the phenomenon of radiation recall dermatitis. Radiother Oncol 2001; 59 (03) 237-245 http://www.ncbi.nlm.nih.gov/pubmed/11369064
- D'Angio GJ, Farber S, Maddock CL. Potentiation of x-ray effects by actinomycin D. Radiology 1959; 73 (02) 175-177
- Bentzen SM, Agrawal RK, Aird EG. et al; START Trialists' Group. The UK Standardisation of Breast Radiotherapy (START) Trial A of radiotherapy hypofractionation for treatment of early breast cancer: a randomised trial. Lancet Oncol 2008; 9 (04) 331-341
- Ben-Yosef H. Radiation recall dermatitis. Lancet 1996; 348 (9037): 1321 http://www.ncbi.nlm.nih.gov/pubmed/8909413
- Guarneri C, Guarneri B. Radiation recall dermatitis. CMAJ 2010; 182 (03) E150-E150
- Cho S, Breedlove JJ, Gunning ST. Radiation recall reaction induced by levofloxacin. J Drugs Dermatol 2008; 7 (01) 64-67 http://www.ncbi.nlm.nih.gov/pubmed/18246700
- Jain S, Agarwal J, Laskar S, Gupta T, Shrivastava S. Radiation recall dermatitis with gatifloxacin: a review of literature. J Med Imaging Radiat Oncol 2008; 52 (02) 191-193
- Le Scodan R, Wyplosz B, Couchon S, Housset M, Laccourreye O. UV-light induced radiation recall dermatitis after a chemoradiotherapy organ preservation protocol. Eur Arch Otorhinolaryngol 2007; 264 (09) 1099-1102
- Ng AWY, Wong FCS, Tung SY. S K O. Nimesulide–a new trigger of radiation recall reaction. Clin Oncol (R Coll Radiol) 2007; 19 (05) 364-365
- Marchand A, Georgin-Mège M, Cellier P, Martin L, Avenel-Audran M, Le Corre Y. Exemestane-induced radiation recall dermatitis and morbilliform rash. J Dermatol 2016; 43 (05) 575-576
- Levy A, Hollebecque A, Bourgier C. et al. Targeted therapy-induced radiation recall. Eur J Cancer 2013; 49 (07) 1662-1668
- Alsabbak H, Aljuboori Z, Spierer M, Klein P. The association of adjuvant trastuzumab (Herceptin) with radiation recall dermatitis: a case study. J Cancer Sci Ther 2013; 05 (12) DOI: 10.4172/1948-5956.1000236.
- Moon D, Koo JS, Suh C-O, Yoon CY, Bae J, Lee S. Radiation recall dermatitis induced by trastuzumab. Breast Cancer 2016; 23 (01) 159-163
- Shrimali RK, McPhail NJ, Correa PD, Fraser J, Rizwanullah M. Trastuzumab-induced radiation recall dermatitis–first reported case. Clin Oncol (R Coll Radiol) 2009; 21 (08) 634-635
- Lee HE, Jeong NJ, Lee Y. et al. Radiation recall dermatitis and pneumonitis induced by trastuzumab (Herceptin®). Int J Dermatol 2014; 53 (03) e159-e160 http://www.ncbi.nlm.nih.gov/pubmed/24716200
- Chung C, Stuart D, Keyes M. Radiation recall reaction induced by adjuvant trastuzumab (Herceptin). Case Rep Med 2009; 2009: 307894 DOI: 10.1155/2009/307894.
- Anupama C, Vinayak V, Anuradha H, Maka V. Trastuzumab induced radiation recall dermatitis: an interesting case. Int J Basic Clin Pharmacol 2018; 7 (12) 2465-2467
- Slamon DJ, Leyland-Jones B, Shak S. et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med 2001; 344 (11) 783-792
- Corbin KS, Breen WG, Strauss JB. Radiation dermatitis in patients treated with concurrent trastuzumab emtansine (T-DM1). Clin Transl Radiat Oncol 2020; 24: 99-101
- Jeter MD, Jänne PA, Brooks S. et al. Gemcitabine-induced radiation recall. Int J Radiat Oncol Biol Phys 2002; 53 (02) 394-400
- Smith KJ, Germain M, Skelton H. Histopathologic features seen with radiation recall or enhancement eruptions. J Cutan Med Surg 2002; 6 (06) 535-540
- Saif MW, Black G, Johnson M, Russo S, Diasio R. Radiation recall phenomenon secondary to capecitabine: possible role of thymidine phosphorylase. Cancer Chemother Pharmacol 2006; 58 (06) 771-775
- Hird AE, Wilson J, Symons S, Sinclair E, Davis M, Chow E. Radiation recall dermatitis: case report and review of the literature. Curr Oncol 2008; 15 (01) 53-62 http://www.ncbi.nlm.nih.gov/pubmed/18317586
- Khanna NR,
Kumar DP, Laskar SG, Laskar S. Radiation dermatitis:
an overview. Indian J Burns 2013; 21 (01) 24 DOI: 10.4103/0971-653X.121877.
Address for correspondence
Rohit Vadgaonkar, MDDepartment of Radiation Oncology, Breast cancer management group, Homi Bhabha Cancer Hospital and Research CentreAPIIC Plot, Aganampudi village, National Highway No.5, Visakhapatnam 530053, Andhra PradeshIndiaEmail: dr.ravad@gmail.comPublication History
Article published online:
12 May 2023© 2023. The Author(s). This is an open access article published by Thieme under the terms of the Creative Commons Attribution License, permitting unrestricted use, distribution, and reproduction so long as the original work is properly cited. (https://creativecommons.org/licenses/by/4.0/)
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India

| Fig 1 :(A) Area of moist desquamation, 7 days post-LRRT. (B) Radiation recall dermatitis (RRD), 14 days post-trastuzumab, 28 days post-LRRT. Reaction was well demarcating radiation chest wall portals. (C) Near complete resolution of RRD with small persistent area of moist desquamation along the scar, 42 days post-LRRT. LRRT, locoregional radiotherapy;
References
- Azria D, Magné N, Zouhair A. et al. Radiation recall: a well recognized but neglected phenomenon. Cancer Treat Rev 2005; 31 (07) 555-570
- Burris III HA, Hurtig J. Radiation recall with anticancer agents. Oncologist 2010; 15 (11) 1227-1237
- Camidge R, Price A. Characterizing the phenomenon of radiation recall dermatitis. Radiother Oncol 2001; 59 (03) 237-245 http://www.ncbi.nlm.nih.gov/pubmed/11369064
- D'Angio GJ, Farber S, Maddock CL. Potentiation of x-ray effects by actinomycin D. Radiology 1959; 73 (02) 175-177
- Bentzen SM, Agrawal RK, Aird EG. et al; START Trialists' Group. The UK Standardisation of Breast Radiotherapy (START) Trial A of radiotherapy hypofractionation for treatment of early breast cancer: a randomised trial. Lancet Oncol 2008; 9 (04) 331-341
- Ben-Yosef H. Radiation recall dermatitis. Lancet 1996; 348 (9037): 1321 http://www.ncbi.nlm.nih.gov/pubmed/8909413
- Guarneri C, Guarneri B. Radiation recall dermatitis. CMAJ 2010; 182 (03) E150-E150
- Cho S, Breedlove JJ, Gunning ST. Radiation recall reaction induced by levofloxacin. J Drugs Dermatol 2008; 7 (01) 64-67 http://www.ncbi.nlm.nih.gov/pubmed/18246700
- Jain S, Agarwal J, Laskar S, Gupta T, Shrivastava S. Radiation recall dermatitis with gatifloxacin: a review of literature. J Med Imaging Radiat Oncol 2008; 52 (02) 191-193
- Le Scodan R, Wyplosz B, Couchon S, Housset M, Laccourreye O. UV-light induced radiation recall dermatitis after a chemoradiotherapy organ preservation protocol. Eur Arch Otorhinolaryngol 2007; 264 (09) 1099-1102
- Ng AWY, Wong FCS, Tung SY. S K O. Nimesulide–a new trigger of radiation recall reaction. Clin Oncol (R Coll Radiol) 2007; 19 (05) 364-365
- Marchand A, Georgin-Mège M, Cellier P, Martin L, Avenel-Audran M, Le Corre Y. Exemestane-induced radiation recall dermatitis and morbilliform rash. J Dermatol 2016; 43 (05) 575-576
- Levy A, Hollebecque A, Bourgier C. et al. Targeted therapy-induced radiation recall. Eur J Cancer 2013; 49 (07) 1662-1668
- Alsabbak H, Aljuboori Z, Spierer M, Klein P. The association of adjuvant trastuzumab (Herceptin) with radiation recall dermatitis: a case study. J Cancer Sci Ther 2013; 05 (12) DOI: 10.4172/1948-5956.1000236.
- Moon D, Koo JS, Suh C-O, Yoon CY, Bae J, Lee S. Radiation recall dermatitis induced by trastuzumab. Breast Cancer 2016; 23 (01) 159-163
- Shrimali RK, McPhail NJ, Correa PD, Fraser J, Rizwanullah M. Trastuzumab-induced radiation recall dermatitis–first reported case. Clin Oncol (R Coll Radiol) 2009; 21 (08) 634-635
- Lee HE, Jeong NJ, Lee Y. et al. Radiation recall dermatitis and pneumonitis induced by trastuzumab (Herceptin®). Int J Dermatol 2014; 53 (03) e159-e160 http://www.ncbi.nlm.nih.gov/pubmed/24716200
- Chung C, Stuart D, Keyes M. Radiation recall reaction induced by adjuvant trastuzumab (Herceptin). Case Rep Med 2009; 2009: 307894 DOI: 10.1155/2009/307894.
- Anupama C, Vinayak V, Anuradha H, Maka V. Trastuzumab induced radiation recall dermatitis: an interesting case. Int J Basic Clin Pharmacol 2018; 7 (12) 2465-2467
- Slamon DJ, Leyland-Jones B, Shak S. et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med 2001; 344 (11) 783-792
- Corbin KS, Breen WG, Strauss JB. Radiation dermatitis in patients treated with concurrent trastuzumab emtansine (T-DM1). Clin Transl Radiat Oncol 2020; 24: 99-101
- Jeter MD, Jänne PA, Brooks S. et al. Gemcitabine-induced radiation recall. Int J Radiat Oncol Biol Phys 2002; 53 (02) 394-400
- Smith KJ, Germain M, Skelton H. Histopathologic features seen with radiation recall or enhancement eruptions. J Cutan Med Surg 2002; 6 (06) 535-540
- Saif MW, Black G, Johnson M, Russo S, Diasio R. Radiation recall phenomenon secondary to capecitabine: possible role of thymidine phosphorylase. Cancer Chemother Pharmacol 2006; 58 (06) 771-775
- Hird AE, Wilson J, Symons S, Sinclair E, Davis M, Chow E. Radiation recall dermatitis: case report and review of the literature. Curr Oncol 2008; 15 (01) 53-62 http://www.ncbi.nlm.nih.gov/pubmed/18317586
- Khanna NR, Kumar DP, Laskar SG, Laskar S. Radiation dermatitis: an overview. Indian J Burns 2013; 21 (01) 24 DOI: 10.4103/0971-653X.121877.


 PDF
PDF  Views
Views  Share
Share

