Rapidly Progressive Pleuro-parenchymal Fibroelastosis Secondary to Cyclophosphamide Chemotherapy
CC BY-NC-ND 4.0 · Indian J Med Paediatr Oncol 2020; 41(03): 427-429
DOI: DOI: 10.4103/ijmpo.ijmpo_85_19
Abstract
Pleuroparenchymal fibroelastosis (PPFE) is a rare progressive interstitial lung disease presenting with predominantly upper-lobe pleural thickening and pulmonary fibrosis. While most cases are idiopathic, it is rarely seen as a complication of infection or following chemotherapy, radiation therapy, and bone marrow transplant. We describe a case of early onset and rapidly progressive PPFE as a complication of cyclophosphamide chemotherapy.
Publication History
Received: 02 April 2019
Accepted: 06 June 2019
Article published online:
28 June 2021
© 2020. Indian Society of Medical and Paediatric Oncology. This is an open access article published by Thieme under the terms of the Creative Commons Attribution-NonDerivative-NonCommercial-License, permitting copying and reproduction so long as the original work is given appropriate credit. Contents may not be used for commercial purposes, or adapted, remixed, transformed or built upon. (https://creativecommons.org/licenses/by-nc-nd/4.0/.)
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India
Abstract
Pleuroparenchymal fibroelastosis (PPFE) is a rare progressive interstitial lung disease presenting with predominantly upper-lobe pleural thickening and pulmonary fibrosis. While most cases are idiopathic, it is rarely seen as a complication of infection or following chemotherapy, radiation therapy, and bone marrow transplant. We describe a case of early onset and rapidly progressive PPFE as a complication of cyclophosphamide chemotherapy.
Introduction
Cyclophosphamide is a commonly used alkylating agent in chemotherapy as well as an immunosuppressant. Cyclophosphamide is activated primarily in the liver to the toxic metabolites acrolein, 4-hydroxycyclophosphamide and phosphoramide mustard. Lung injury in the form of pneumonitis is due to increased transforming growth factor-β production and enhanced collagen synthesis.[1] Few reports describe the development of pleuroparenchymal fibroelastosis (PPFE) over 18–72 months after the initiation of cyclophosphamide therapy which usually progresses gradually.[2]
An interesting case of early-onset and rapidly progressive PPFE induced by cyclophosphamide is described below.
Case Report
A 16-year-old boy diagnosed with primitive neuroectodermal tumor (PNET) of the chest wall (right second rib) treated with surgery (local excision), focal radiotherapy, and systemic chemotherapy (vincristine, doxorubicin, ifosfamide, etoposide, cyclophosphamide, and dactinomycin) in 2012. After a disease-free interval of 4 years, he had disease recurrence in the form of solitary right upper-lobe pulmonary nodule which was excised. This was followed by concurrent chemotherapy(consisting of topotecan, cyclophosphamide, temozolomide and irinotecan) and radiotherapy (consisting of bilateral lung irradiation of 11.2 Gray in 7 fractions). After completion of 8 months of above-mentioned salvage chemotherapy, he was started on oral maintenance chemotherapy (cyclophosphamide, etoposide, celecoxib, and tamoxifen). Six months later, on this oral chemotherapy regimen, the patient developed mild exertional breathlessness, dry cough, and fatigue. High-resolution computed tomography (HRCT) of the chest revealed the presence of bronchocentric and diffuse ground-glass opacities predominantly in bilateral upper lobes [Figure 1]. The patient was afebrile. Respiratory rate was 16 breaths/min with a saturation of 97%-on room air. Normal vesicular breath sounds were present on auscultation. He had a normal hemogram and bronchoscopy with bronchoalveolar lavage was negative for Pneumocystis jirovecii, bacterial and tuberculous infection. The patient was initiated on therapy with oral corticosteroids; however, he had no symptomatic relief and developed worsening of breathlessness over the next 4 months. His resting respiratory rate increased to 26/min, and few fine end-inspiratory crackles were noted in bilateral infraclavicular areas on auscultation. Six-minute walk test (6MWT) at this assessment showed desaturation from 95% to 86%. Bilateral upper-lobe pleural thickening with subpleural fibrosis was noted in repeat HRCT chest [Figure 2] in the same areas which previously had ground glass opacities. In addition, bilateral loculated apical pneumothoraces with minimal fibrosis in lower lobes were noted suggestive of PPFE.
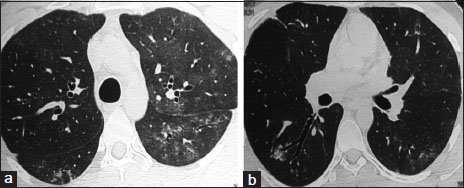
| Figure 1:(a and b) High-resolution computed tomography chest showing centrilobular ground glass opacities in bilateral upper lobes and superior basal segments
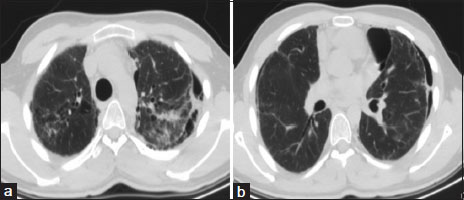
| Figure.2:(a and b) High-resolution computed tomography chest showing bilateral upper-lobe interseptal thickening with pleural thickening and pneumothorax
Discussion
The entity of idiopathic upper-lobe pulmonary fibrosis was first described in 13 patients by Amitani et al. in 1992. Frankel et al. are credited with coining the term “pleuropulmonary fibroelastosis” in 2004.[3] Idiopathic PPFE has subsequently been classified as a separate entity under rare idiopathic interstitial pneumonias.[4]
PPFE is a rare progressive interstitial lung disease manifesting with predominantly upper-lobe pleural thickening and subpleural fibrosis. Patients may remain asymptomatic for a while; however, those that progress are often complicated by the presence of pneumothorax. Most cases are idiopathic with bimodal age distribution at 20–30 years and 50–60 years, with a slender flat chest cage and usually minimal findings on clinical examination.[3] Histologically, evolution of PPFE has been described as initially presenting with either interstitial inflammation with fibrosis or as organizing pneumonia pattern which progresses to subpleural intraalveolar fibrosis with elastosis of the walls and patchy lymphoplasmacytic infiltrates. In addition, the presence of upper zone fibrosis of visceral pleura and small numbers of fibroblastic foci help to achieve a definitive diagnosis on histology.[5]
Secondary causes of PPFE described in literature include systemic chemotherapeutic agents such as carmustine and cyclophosphamide, radiation therapy (more than 20 Gray), and bone marrow transplant.[2],[6],[7]
Clinically, patients of PPFE develop progressive exertional breathlessness with restrictive defect and impaired diffusion capacity on lung functions along with exercise-induced desaturation. Our patient was too breathless to test for lung functions; however, his desaturation at 6MWT was suggestive of ventilatory impairment.
In PPFE, subpleural nodular and reticular opacities in the upper lobes with minimal involvement of the other lobes are noted in initial stages of HRCT of the lung. Later in progressive disease, pleuroparenchymal thickening of 4–15 mm with fibrosis and volume loss, traction bronchiectasis with bullae and occasionally large cysts in upper lobes with pneumothorax are described.[8]
Reddy et al.[8] have put forth definitive and consistent criteria for the diagnosis of PPFE on the basis of imaging as follows:
Definitive pleuroparenchymal fibroelastosis
Presence of upper-lobe pleural thickening with subpleural fibrosis with absent or minimal fibrosis in lower lobes.
Diagnosis consistent with pleuroparenchymal fibroelastosis
Presence of upper-lobe pleural thickening and subpleural fibrosis that are not concentrated in the upper lobe or presence of coexistent disease elsewhere.
In the presented case, the initial radiological features of ground glass opacities predominantly in upper lobes with subsequent development of bilateral upper-lobe fibrosis and pleural thickening fulfill the criteria for definitive diagnosis of PPFE.
Systemic cyclophosphamide-induced lung toxicity is classified into early and late pneumonitis as described by Malik et al. Early-onset pneumonitis develops within 6 months of initiation of therapy and may be reversible with withdrawal of offending agent and treatment with steroids. It is characterized by the presence of interlobular septal thickening and ground glass appearance showing no lobar predilection on HRCT. Late-onset pneumonitis has been reported to occur over 18 months–12 years after initiation of therapy with cyclophosphamide and is generally not responsive to steroids.[9]
The causality of PPFE due to cyclophosphamide in this case has been established due to the onset of symptoms 14 months after initiation of chemotherapy with cyclophosphamide which includes 8 months of salvage and 6 months of oral maintenance chemotherapy. It is unlikely that the initial radiological manifestation is radiation-induced since radiation-induced pneumonitis occurs usually within 2–3 months of radiation, with a radiation dose of more than 20 Gray and high V20 (volume of lung receiving 20 Gray or more). Our patient received only a low dose 11.2 Gray of radiation 9 months before the development of disease. Normal lung imaging, barring the right upper-lobe PNET at disease recurrence, obviates the contribution of prior chemoradiotherapy toward the development of current PPFE.
The time interval between the onset of interstitial inflammation to progression to pulmonary fibrosis described in literature is between 31 months and 12 years.[5] Despite treatment with steroids, in this case, there was a rapid progression to the fibrotic phase of PPFE from pneumonitis within 4 months.
Conclusion
PPFE with pneumothorax has a poor prognosis with poor response to steroids. A single case of successful living donor lung transplant for PPFE is described in literature.[10] Due to its relentless progressive nature causing crippling respiratory disability, physicians need to be aware of the occurrence of this disease in patients on cyclophosphamide therapy. While the restrictive versus liberal fluid therapy for major abdominal surgery trial is ongoing for the role of antifibrotics in conditions other than idiopathic pulmonary fibrosis, perhaps the role of antifibrotics such as pirfenidone in PPFE is a subject for future research.[11]
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Acknowledgment
We would like to thank Dr. Pavan Biraris, Consultant, Department of Pulmonary Medicine, Tata Memorial Hospital.
Conflict of Interest
There are no conflicts of interest.
References
- Hoyt DG, Lazo JS. Early increases in pulmonary mRNA encoding procollagens and transforming growth factor-beta in mice sensitive to cyclophosphamide-induced pulmonary fibrosis. J Pharmacol Exp Ther 1989; 249: 38-43
- Watanabe K. Pleuroparenchymal fibroelastosis: Its clinical characteristics. Curr Respir Med Rev 2013; 9: 299-237
- Frankel SK, Cool CD, Lynch DA, Brown KK. Idiopathic pleuroparenchymal fibroelastosis: Description of a novel clinicopathologic entity. Chest 2004; 126: 2007-13
- Travis WD, Costabel U, Hansell DM, King TEJr, Lynch DA, Nicholson AG. et al. An official American thoracic society/European respiratory society statement: Update of the international multidisciplinary classification of the idiopathic interstitial pneumonias. Am J Respir Crit Care Med 2013; 188: 733-48
- Hirota T, Yoshida Y, Kitasato Y, Yoshimi M, Koga T, Tsuruta N. et al. Histological evolution of pleuroparenchymal fibroelastosis. Histopathology 2015; 66: 545-54
- Beynat-Mouterde C, Beltramo G, Lezmi G, Pernet D, Camus C, Fanton A. et al. Pleuroparenchymal fibroelastosis as a late complication of chemotherapy agents. Eur Respir J 2014; 44: 523-7
- von der ThüsenJH, Hansell DM, Tominaga M, Veys PA, Ashworth MT, Owens CM. et al. Pleuroparenchymal fibroelastosis in patients with pulmonary disease secondary to bone marrow transplantation. Mod Pathol 2011; 24: 1633-9
- Reddy TL, Tominaga M, Hansell DM, von der ThusenJ, Rassl D, Parfrey H. et al. Pleuroparenchymal fibroelastosis: A spectrum of histopathological and imaging phenotypes. Eur Respir J 2012; 40: 377-85
- Malik SW, Myers JL, DeRemee RA, Specks U. Lung toxicity associated with cyclophosphamide use. Two distinct patterns. Am J Respir Crit Care Med 1996; 154: 1851-6
- Hata A, Nakajima T, Yoshida S, Kinoshita T, Terada J, Tatsumi K. et al. Living donor lung transplantation for pleuroparenchymal fibroelastosis. Ann Thorac Surg 2016; 101: 1970-2
- Behr J, Neuser P, Prasse A, Kreuter M, Rabe K, Schade-Brittinger C. et al. Exploring efficacy and safety of oral pirfenidone for progressive, non-IPF lung fibrosis (RELIEF) – A randomized, double-blind, placebo-controlled, parallel group, multi-center, phase II trial. BMC Pulm Med 2017; 17: 122
Address for correspondence
Publication History
Received: 02 April 2019
Accepted: 06 June 2019
Article published online:
28
June 2021
© 2020. Indian Society of Medical and Paediatric Oncology. This is an open access article published by Thieme under the terms of the Creative Commons Attribution-NonDerivative-NonCommercial-License, permitting copying and reproduction so long as the original work is given appropriate credit. Contents may not be used for commercial purposes, or adapted, remixed, transformed or built upon. (https://creativecommons.org/licenses/by-nc-nd/4.0/.)
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2, Noida-201301
UP, India

| Figure 1:(a and b) High-resolution computed tomography chest showing centrilobular ground glass opacities in bilateral upper lobes and superior basal segments

| Figure.2:(a and b) High-resolution computed tomography chest showing bilateral upper-lobe interseptal thickening with pleural thickening and pneumothorax
References
- Hoyt DG, Lazo JS. Early increases in pulmonary mRNA encoding procollagens and transforming growth factor-beta in mice sensitive to cyclophosphamide-induced pulmonary fibrosis. J Pharmacol Exp Ther 1989; 249: 38-43
- Watanabe K. Pleuroparenchymal fibroelastosis: Its clinical characteristics. Curr Respir Med Rev 2013; 9: 299-237
- Frankel SK, Cool CD, Lynch DA, Brown KK. Idiopathic pleuroparenchymal fibroelastosis: Description of a novel clinicopathologic entity. Chest 2004; 126: 2007-13
- Travis WD, Costabel U, Hansell DM, King TEJr, Lynch DA, Nicholson AG. et al. An official American thoracic society/European respiratory society statement: Update of the international multidisciplinary classification of the idiopathic interstitial pneumonias. Am J Respir Crit Care Med 2013; 188: 733-48
- Hirota T, Yoshida Y, Kitasato Y, Yoshimi M, Koga T, Tsuruta N. et al. Histological evolution of pleuroparenchymal fibroelastosis. Histopathology 2015; 66: 545-54
- Beynat-Mouterde C, Beltramo G, Lezmi G, Pernet D, Camus C, Fanton A. et al. Pleuroparenchymal fibroelastosis as a late complication of chemotherapy agents. Eur Respir J 2014; 44: 523-7
- von der ThüsenJH, Hansell DM, Tominaga M, Veys PA, Ashworth MT, Owens CM. et al. Pleuroparenchymal fibroelastosis in patients with pulmonary disease secondary to bone marrow transplantation. Mod Pathol 2011; 24: 1633-9
- Reddy TL, Tominaga M, Hansell DM, von der ThusenJ, Rassl D, Parfrey H. et al. Pleuroparenchymal fibroelastosis: A spectrum of histopathological and imaging phenotypes. Eur Respir J 2012; 40: 377-85
- Malik SW, Myers JL, DeRemee RA, Specks U. Lung toxicity associated with cyclophosphamide use. Two distinct patterns. Am J Respir Crit Care Med 1996; 154: 1851-6
- Hata A, Nakajima T, Yoshida S, Kinoshita T, Terada J, Tatsumi K. et al. Living donor lung transplantation for pleuroparenchymal fibroelastosis. Ann Thorac Surg 2016; 101: 1970-2
- Behr J, Neuser P, Prasse A, Kreuter M, Rabe K, Schade-Brittinger C. et al. Exploring efficacy and safety of oral pirfenidone for progressive, non-IPF lung fibrosis (RELIEF) – A randomized, double-blind, placebo-controlled, parallel group, multi-center, phase II trial. BMC Pulm Med 2017; 17: 122


 PDF
PDF  Views
Views  Share
Share

