Renal Cell Cancer: Clinicopathological Profile and Survival Outcomes
CC BY-NC-ND 4.0 ? Indian J Med Paediatr Oncol 2018; 39(01): 23-27
DOI: DOI: 10.4103/ijmpo.ijmpo_126_16
Abstract
Background:?The incidence of renal cell cancer (RCC) is increasing worldwide. However, scant information is available from the Indian subcontinent regarding its clinicopathological characteristics and survival outcomes. We retrospectively analyzed data of patients suffering from RCC at our center over the last one decade (2004?2013) to generate information on these aspects.?Materials and Methods:?Case records of 423 patients treated between 2004 and 2013 were retrospectively analyzed. Baseline characteristics, histopathological information, and survival outcomes were assessed. Overall survival was calculated from the time of diagnosis to death due to any cause.?Results:?The median age was 52 years (range: 18?87 years). Male: female ratio was 3.5:1. The median duration of symptoms was 3 months (range: 0?24 months). Thirty-five patients (8.3%) were detected in asymptomatic state. The most common symptom was hematuria (53.2%) followed by flank pain (46.3%). The most common histology was clear cell subtype (71.4%). Two hundred and ninety-three (69.3%) patients presented with nonmetastatic disease whereas 130 (30.7%) had upfront metastatic disease. Five-year survival in Stages 1, 2, 3, and 4 was 92.7%, 72.9%, 54.6%, and 11.5%, respectively.?Conclusion:?Younger age, higher male?female ratio, lower proportion of asymptomatic patients, higher proportion of advanced stage at diagnosis, and lower stage-wise survival were some of the key findings.
Keywords
Clear cell - hematuria - renal cell cancer
Publication History
23 June 2021 (online)
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India
Abstract
Background:?The incidence of renal cell cancer (RCC) is increasing worldwide. However, scant information is available from the Indian subcontinent regarding its clinicopathological characteristics and survival outcomes. We retrospectively analyzed data of patients suffering from RCC at our center over the last one decade (2004?2013) to generate information on these aspects.?Materials and Methods:?Case records of 423 patients treated between 2004 and 2013 were retrospectively analyzed. Baseline characteristics, histopathological information, and survival outcomes were assessed. Overall survival was calculated from the time of diagnosis to death due to any cause.?Results:?The median age was 52 years (range: 18?87 years). Male: female ratio was 3.5:1. The median duration of symptoms was 3 months (range: 0?24 months). Thirty-five patients (8.3%) were detected in asymptomatic state. The most common symptom was hematuria (53.2%) followed by flank pain (46.3%). The most common histology was clear cell subtype (71.4%). Two hundred and ninety-three (69.3%) patients presented with nonmetastatic disease whereas 130 (30.7%) had upfront metastatic disease. Five-year survival in Stages 1, 2, 3, and 4 was 92.7%, 72.9%, 54.6%, and 11.5%, respectively.?Conclusion:?Younger age, higher male?female ratio, lower proportion of asymptomatic patients, higher proportion of advanced stage at diagnosis, and lower stage-wise survival were some of the key findings.
Keywords
Clear cell - hematuria - renal cell cancer
Introduction
Renal cell cancer (RCC) is an uncommon malignancy. It constitutes <3 href="https://www.thieme-connect.com/products/ejournals/html/10.4103/ijmpo.ijmpo_126_16#OR_1" xss=removed>1] Nonetheless, incidence is currently increasing at a rate of 2% per year in many developed countries.[2],[3] Its frequency is also expected to rise in India due to increasing life expectancy, rising awareness, better diagnostic facilities, and growing prevalence of risk factors such as obesity.[4] However, there is a paucity of data for RCC from the Indian subcontinent.[5],[6] Hence, to generate more information on Indian cohort of RCC, we retrospectively analyzed data of 423 consecutive patients treated over 10 years.
Materials and Methods
We collected data of patients registered with histopathological diagnosis of RCC from January 2004 to December 2013 from hospital case records. Clinical and laboratory parameters were entered in the predesigned pro forma. Clinical parameters assessed included age, sex, place of residence, family history, occupation, history of smoking, comorbidities, presenting complaints and duration of illness, stage at the time of diagnosis, duration of follow-up, and time of death. Histopathological parameters included subtypes of RCC, Fuhrman grading, and tumor and lymph nodal staging. Follow-up details were gathered from the date of last outpatient visit, telephonic inquiry, or with the help of a reply postcard. The primary end point was overall survival (OS), calculated from the time of diagnosis to death due to any cause.
Statistical analysis
Descriptive statistics were used to describe demographic and clinical characteristics. Kaplan?Meier method was used to estimate OS. All analyses were performed using SPSS version 20 statistical software (SPSS Inc., Chicago, Illinois, USA). Data were censored on December 31, 2014, or on the date of last follow-up.
Results
Overall, 477 patients were registered with the diagnosis of RCC at our center between January 2004 and December 2013. Fifty-four individuals were excluded due to lack of sufficient information regarding baseline parameters. Out of the 423 patients included in the analysis, data regarding OS were available for 331 (78.3%) patients. [Table 1] lists various baseline characteristics and associated comorbidities. Maximum patients belonged to the age group of 51?60 years [Figure 1]. Twenty-four (4.7%) and 85 (20.1%) patients were <30>
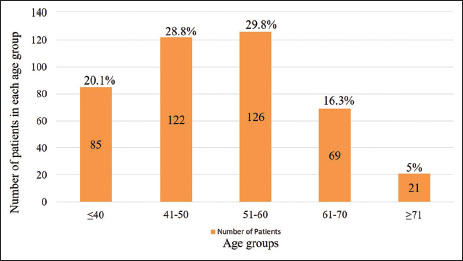
|?Figure 1Age distribution of patients
|
Parameter |
n (%) |
|---|---|
|
BPH ? Benign prostatic hyperplasia; COPD ? Chronic obstructive pulmonary disease |
|
|
Age (years), median (range) |
52 (18-87) |
|
Sex |
|
|
Male |
330(78) |
|
Female |
93 (22) |
|
History of smoking |
181 (42.8) |
|
Comorbidities |
|
|
Hypertension |
115 (27.2) |
|
Diabetes mellitus |
62 (14.9) |
|
Coronary artery disease |
23 (5.5) |
|
BPH |
28 (8.4) |
|
Hypothyroidism |
14 (3.4) |
|
Bronchial asthma/COPD |
11 (2.7) |
|
Cerebrovascular disease |
8(1.9) |
|
Others |
16 (3.8) |
|
Clinical features |
|
|
Hematuria |
225 (53.2) |
|
Flank pain |
196 (46.3) |
|
Weight loss |
139 (32.9) |
|
Lump abdomen |
114 (22.5) |
|
Fever |
81 (19.5) |
|
Loss of appetite |
41 (9.9) |
|
Bone pains |
33 (8.0) |
|
Cough |
31 (7.7) |
|
Shortness of breath |
28 (7.9) |
|
Varicocele |
10 (2.4) |
|
Triad of hematuria, lump, and flank pain |
84 (20.2) |
|
n (%) |
|
|---|---|
|
Histopathology |
|
|
Clear cell |
302 (71.4) |
|
Papillary type 1 |
42 (9.9) |
|
Papillary type 2 |
25 (5.9) |
|
Chromophobe |
4 (3.5) |
|
Oncocytoma |
6 (1.4) |
|
Collecting duct |
6 (1.4) |
|
Sarcomatoid/rhabdoid |
10 (2.4) |
|
Unclassified |
14 (3.3) |
|
Others |
4 (1) |
|
Tumor stage |
|
|
T1a |
36 (12.5) |
|
T1b |
52 (18) |
|
T2a |
49 (17) |
|
T2b |
37 (12.8) |
|
T3a |
70 (24.2) |
|
T3b |
28 (9.7) |
|
T3c |
6 (2.1) |
|
T4 |
12 (4.2) |
|
Site of metastases |
|
|
Lungs |
77 (59.2) |
|
Bones |
68 (51.9) |
|
Distant lymph nodes |
41 (31.5) |
|
Liver |
33 (25.4) |
|
Soft tissue |
21 (16.2) |
|
Adrenals |
18 (13.9) |
|
Brain |
13(10) |
|
Skin |
7 (5.4) |
|
Other sites |
21 (16.2) |
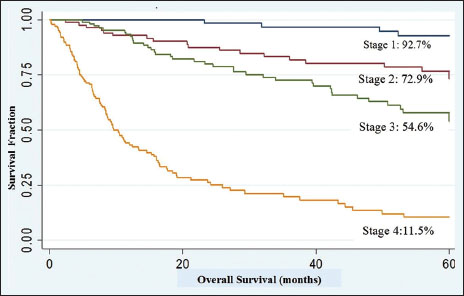
|?Figure 2Five-year overall survival according to various stages
Discussion
In this analysis, we evaluated the various clinical and histopathological parameters and survival outcomes from a tertiary care center. Median age in our study was 52 years. This is almost a decade lower compared to the median age of 62?64 years reported in studies from Europe and North America.[2] Other Indian studies have also shown similar age pattern.[7] Precise mechanisms for this age difference are unclear. The population pyramid in India constitutes of higher proportion of younger population as compared to the West, and this might be one of the reasons for younger age at presentation. Importantly, 24 patients (4.7%) were <30>
Male-to-female ratio was 3.5:1. Many reports have revealed higher male representation for RCC.[8] The strongest risk factor for RCC is smoking. In many countries, smoking is more common in males than females and this might account for higher proportion of men for RCC.[9] In India also, the frequency of smoking in men is much higher than that of women.[10] Besides, some studies have hypothesized hormonal influences including protective effect of oral contraceptive pills for the lower incidence of RCC in women.[11],[12]
The most common comorbidities detected were hypertension and type 2 diabetes mellitus. Hypertension is one of the established risk factors as well as a paraneoplastic manifestation of RCC.[13],[14] In addition, it has a bearing on the treatment. Anti-angiogenesis therapy, currently the standard of care in metastatic RCC, leads to worsening of hypertension in many patients. This converts into more outpatient visits, higher pill burden by addition of multiple antihypertensive drugs, and increased rate of treatment discontinuation. Patients of diabetes and/or hypertension have propensity to develop chronic kidney disease with radical nephrectomy and hence should undergo functional scans prior to nephrectomy. Partial nephrectomy should be encouraged in such patients.
Two patients had RCC developing in native kidney after renal transplantation. Both these patients were on strong immunosuppressive drugs. RCC of native end-stage kidneys is found in about 4% of patients. End-stage renal disease as well as immunosuppression are risk factors for RCC.[15],[16],[17] The lifetime risk of developing RCC in this group is at least 10 times larger than in the general population.[16] In addition, these patients have aggressive disease and dismal outcomes.[17]
Approximately 8.3% of patients were detected in asymptomatic condition. This figure is markedly lower than reports from North America and Europe.[2],[18] In India, thresholds to use abdominal imaging for any indication are higher due to lack of affordability and limited availability. Hence, India is still to see the stage migration of RCC, being seen in the West.[19] The most common manifestation was hematuria followed by flank pain and weight loss. Interestingly, 20% of patients presented with the conventional triad of hematuria, flank pain, and palpable lump in abdomen. Occurrence of this triad depicts advanced disease. Previous descriptions report its frequency in not> 10% of patients.[20] It reflects greater percentage of patients presenting with advanced disease in our study.
Clear cell carcinoma was the most common histological subtype followed by papillary type I. These findings are similar to that described in literature.[21] Fuhrman Grade 2 tumors were most common followed by Grade 3. Importantly, there were 16% of patients with Grade 4 tumors which is higher than reported in other retrospective studies.[22] Pure sarcomatoid subtype was detected in nine patients. It denotes aggressive disease and is associated with poor survival outcomes.[23] Two patients had mucinous, tubular, and spindle cell tumor. It is a recently described entity, seen more commonly in females and considered to be a low-grade renal malignancy.[24] In our study, both patients with this subtype were females with early-stage tumor.
Stage 4 and 3 diseases were more common than Stage 1 and 2 diseases at the time of diagnosis. Majority of studies are currently reporting Stage 1 disease in> 50% of patients due to a greater number of asymptomatic detection.[19] This finding has implications on deciding the line of management and survival outcomes as stage is the most important independent prognostic factor. Furthermore, in our study, higher number of patients presented with thrombus formation. Other than determining stage and prognosis, thrombus removal demands greater surgical expertise and more invasive surgery.
Five-year survival in the study for Stage 1 was equivalent to those reported elsewhere, however 5-year OS for Stages 2, 3, and 4 was lower.[2],[25],[26] Five-year OS was 55.1%. This figure is again low compared to many other analyses that have revealed 5-year OS> 60%.[26],[27] Few causes can be deciphered for this. First, higher percentage of our patients had Fuhrman Grade 3 and 4 diseases (49.2%) which is an established maker of aggressive disease. Second, in Stage 3, higher number of patients had 3b and 3c Stage due to inferior vena cava (IVC) involvement.[28] Any kind of IVC involvement adversely affects prognosis.[29] Third, in the metastatic setting, many patients were not able to afford targeted therapies, lowering survival in Stage 4 disease.
Important limitations in the present study are: first, we did not have follow-up details regarding OS in approximately 22% of patients. Often patients change their contact numbers that do not get updated in the record system and hence could not be traced after stopping outpatient visits. Second, there was missing data regarding body mass index, therefore we could not document the prevalence of obesity in our patients which is a proven risk factor for RCC. Similarly, occupation history was also missing from many case records and consequently no consistent occupational exposure could be found. Last but not the least, this analysis included patients from a single tertiary center, causing potential biases in patient population.
Conclusion
Younger patient population, higher male: female ratio, fewer asymptomatic detection, and higher stage at the time of diagnosis were characteristic features of RCC in this study. Stage-wise survival was inferior in our patients. Though some causes can be deciphered for low survival, we need to have prospective studies to characterize disease biology and validate risk factors.
Conflict of Interest
There are no conflicts of interest.
References
- ancer Facts And Figures 2015. American Cancer Society. Available from: http://www.cancer.org/acs/groups/content/@editorial/documents/document/acspc44552.pdf. [Last accessed on 2016 Jun 02]
- owlader N, Noone AM, Krapcho M, Miller D, Bishop K, Altekruse SF.?et al. SEER Cancer Statistics Review, 1975-2013. Bethesda, MD: National Cancer Institute; 2016. SEER data submission, posted to the SEER web site. Available from: https://seer.cancer.gov/csr/1975_2013/. [Last based on 2016 Jan 12].
- indblad P.?Epidemiology of renal cell carcinoma. Scand J Surg 2004; 93: 88-96
- handelwal S, Reddy KS.?Eliciting a policy response for the rising epidemic of overweight-obesity in India. Obes Rev 2013; 14 Suppl 2: 114-25
- gnihotri S, Kumar J, Jain M, Kapoor R, Mandhani A.?Renal cell carcinoma in India demonstrates early age of onset and a late stage of presentation. Indian J Med Res 2014; 140: 624-9
- rivastava A, Mandhani A, Kapoor R, Jain M, Dubey D, Srivastava A.?et al.?Prognostic factors in patients with renal cell carcinoma: Is TNM (1997) staging relevant in Indian subpopulation?. Indian J Cancer 2004; 41: 99-103
- ivaramakrishna B, Gupta NP, Wadhwa P, Hemal AK, Dogra PN, Seth A.?et al.?Pattern of metastases in renal cell carcinoma: A single institution study. Indian J Cancer 2005; 42: 173-7
- chips L, Lipsky K, Zigeuner R, Salfellner M, Winkler S, Langner C.?et al.?Impact of tumor-associated symptoms on the prognosis of patients with renal cell carcinoma: A single-center experience of 683 patients. Urology 2003; 62: 1024-8
- aldron I, Bratelli G, Carriker L, Sung WC, Vogeli C, Waldman E.?et al.?Gender differences in tobacco use in Africa, Asia, the Pacific, and Latin America. Soc Sci Med 1988; 27: 1269-75
- Rani M, Bonu S, Jha P, Nguyen SN, Jamjoum L.?Tobacco use in India: Prevalence and predictors of smoking and chewing in a national cross sectional household survey. Tob Control 2003; 12: e4
- Chow WH, McLaughlin JK, Mandel JS, Blot WJ, Niwa S, Fraumeni JFJr.?et al.?Reproductive factors and the risk of renal cell cancer among women. Int J Cancer 1995; 60: 312-4
- Mellemgaard A, Engholm G, McLaughlin JK, Olsen JH.?Risk factors for renal-cell carcinoma in Denmark. III Role of weight, physical activity and reproductive factors. Int J Cancer 1994; 56: 66-71
- Chow WH, Gridley G, Fraumeni JFJr, J?rvholm B.?Obesity, hypertension, and the risk of kidney cancer in men. N Engl J Med 2000; 343: 1305-11
- Sufrin G, Chasan S, Golio A, Murphy GP.?Paraneoplastic and serologic syndromes of renal adenocarcinoma. Semin Urol 1989; 7: 158-71
- Lin HF, Li YH, Wang CH, Chou CL, Kuo DJ, Fang TC.?et al.?Increased risk of cancer in chronic dialysis patients: A population-based cohort study in Taiwan. Nephrol Dial Transplant 2012; 27: 1585-90
- Maisonneuve P, Agodoa L, Gellert R, Stewart JH, Buccianti G, Lowenfels AB.?et al.?Cancer in patients on dialysis for end-stage renal disease: An international collaborative study. Lancet 1999; 354: 93-9
- Hora M, Hes O, Reischig T, Urge T, Klecka J, Ferda J.?et al.?Tumours in end-stage kidney. Transplant Proc 2008; 40: 3354-8
- Patard JJ, Rodriguez A, Rioux-Leclercq N, Guill? F, Lobel B.?Prognostic significance of the mode of detection in renal tumours. BJU Int 2002; 90: 358-63
- Kane CJ, Mallin K, Ritchey J, Cooperberg MR, Carroll PR.?Renal cell cancer stage migration: Analysis of the national cancer data base. Cancer 2008; 113: 78-83
- Lee CT, Katz J, Fearn PA, Russo P.?Mode of presentation of renal cell carcinoma provides prognostic information. Urol Oncol 2002; 7: 135-40
- Eble JN, Sauter G, Epstein JI, Sesterhenn IA. editors?In: Pathology and Genetics of Tumors of the Urinary System and Male Genital Organs. World Health Organization Classification of Tumors. Lyons: IARC Press 2004; p: 7
- Fuhrman SA, Lasky LC, Limas C.?Prognostic significance of morphologic parameters in renal cell carcinoma. Am J Surg Pathol 1982; 6: 655-63
- Kanamaru H, Sasaki M, Miwa Y, Akino H, Okada K.?Prognostic value of sarcomatoid histology and volume-weighted mean nuclear volume in renal cell carcinoma. BJU Int 1999; 83: 222-6
- Srigley JR, Delahunt B.?Uncommon and recently described renal carcinomas. Mod Pathol 2009; 22 Suppl 2: S2-23
- Lam JS, Klatte T, Breda A.?Staging of renal cell carcinoma: Current concepts. Indian J Urol 2009; 25: 446-54
- Takashi M, Nakano Y, Sakata T, Miyake K, Hamajima N.?Multivariate evaluation of prognostic determinants for renal cell carcinoma. Urol Int 1993; 50: 6-12
- Wang Y, Huang C, Wu Y, Gao G, Xin Y, Lin Z.?et al.?Multivariate analysis of prognostic factors in renal cell carcinoma. Zhonghua Wai Ke Za Zhi 2000; 38: 442-4
- Mootha RK, Butler R, Laucirica R, Scardino PT, Lerner SP.?Renal cell carcinoma with an infrarenal vena caval tumor thrombus. Urology 1999; 54: 561
- Wagner B, Patard JJ, M?jean A, Bensalah K, Verhoest G, Zigeuner R.?et al.?Prognostic value of renal vein and inferior vena cava involvement in renal cell carcinoma. Eur Urol 2009; 55: 452-9
Address for correspondence
Publication History
Publication Date:
23 June
2021 (online)
? 2018. Indian Society of Medical and Paediatric Oncology. This is an open access article published by Thieme under the terms of the Creative Commons Attribution-NonDerivative-NonCommercial-License, permitting copying and reproduction so long as the original work is given appropriate credit. Contents may not be used for commercial purposes, or adapted, remixed, transformed or built upon. (https://creativecommons.org/licenses/by-nc-nd/4.0/.)
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12,
2nd Floor, Sector 2, Noida-201301 UP, India

|?Figure.1Age distribution of patients

|?Figure.2Five-year overall survival according to various stages
References
- ancer Facts And Figures 2015. American Cancer Society. Available from: http://www.cancer.org/acs/groups/content/@editorial/documents/document/acspc44552.pdf. [Last accessed on 2016 Jun 02]
- owlader N, Noone AM, Krapcho M, Miller D, Bishop K, Altekruse SF.?et al. SEER Cancer Statistics Review, 1975-2013. Bethesda, MD: National Cancer Institute; 2016. SEER data submission, posted to the SEER web site. Available from: https://seer.cancer.gov/csr/1975_2013/. [Last based on 2016 Jan 12].
- indblad P.?Epidemiology of renal cell carcinoma. Scand J Surg 2004; 93: 88-96
- handelwal S, Reddy KS.?Eliciting a policy response for the rising epidemic of overweight-obesity in India. Obes Rev 2013; 14 Suppl 2: 114-25
- gnihotri S, Kumar J, Jain M, Kapoor R, Mandhani A.?Renal cell carcinoma in India demonstrates early age of onset and a late stage of presentation. Indian J Med Res 2014; 140: 624-9
- rivastava A, Mandhani A, Kapoor R, Jain M, Dubey D, Srivastava A.?et al.?Prognostic factors in patients with renal cell carcinoma: Is TNM (1997) staging relevant in Indian subpopulation?. Indian J Cancer 2004; 41: 99-103
- ivaramakrishna B, Gupta NP, Wadhwa P, Hemal AK, Dogra PN, Seth A.?et al.?Pattern of metastases in renal cell carcinoma: A single institution study. Indian J Cancer 2005; 42: 173-7
- chips L, Lipsky K, Zigeuner R, Salfellner M, Winkler S, Langner C.?et al.?Impact of tumor-associated symptoms on the prognosis of patients with renal cell carcinoma: A single-center experience of 683 patients. Urology 2003; 62: 1024-8
- aldron I, Bratelli G, Carriker L, Sung WC, Vogeli C, Waldman E.?et al.?Gender differences in tobacco use in Africa, Asia, the Pacific, and Latin America. Soc Sci Med 1988; 27: 1269-75
- Rani M, Bonu S, Jha P, Nguyen SN, Jamjoum L.?Tobacco use in India: Prevalence and predictors of smoking and chewing in a national cross sectional household survey. Tob Control 2003; 12: e4
- Chow WH, McLaughlin JK, Mandel JS, Blot WJ, Niwa S, Fraumeni JFJr.?et al.?Reproductive factors and the risk of renal cell cancer among women. Int J Cancer 1995; 60: 312-4
- Mellemgaard A, Engholm G, McLaughlin JK, Olsen JH.?Risk factors for renal-cell carcinoma in Denmark. III Role of weight, physical activity and reproductive factors. Int J Cancer 1994; 56: 66-71
- Chow WH, Gridley G, Fraumeni JFJr, J?rvholm B.?Obesity, hypertension, and the risk of kidney cancer in men. N Engl J Med 2000; 343: 1305-11
- Sufrin G, Chasan S, Golio A, Murphy GP.?Paraneoplastic and serologic syndromes of renal adenocarcinoma. Semin Urol 1989; 7: 158-71
- Lin HF, Li YH, Wang CH, Chou CL, Kuo DJ, Fang TC.?et al.?Increased risk of cancer in chronic dialysis patients: A population-based cohort study in Taiwan. Nephrol Dial Transplant 2012; 27: 1585-90
- Maisonneuve P, Agodoa L, Gellert R, Stewart JH, Buccianti G, Lowenfels AB.?et al.?Cancer in patients on dialysis for end-stage renal disease: An international collaborative study. Lancet 1999; 354: 93-9
- Hora M, Hes O, Reischig T, Urge T, Klecka J, Ferda J.?et al.?Tumours in end-stage kidney. Transplant Proc 2008; 40: 3354-8
- Patard JJ, Rodriguez A, Rioux-Leclercq N, Guill? F, Lobel B.?Prognostic significance of the mode of detection in renal tumours. BJU Int 2002; 90: 358-63
- Kane CJ, Mallin K, Ritchey J, Cooperberg MR, Carroll PR.?Renal cell cancer stage migration: Analysis of the national cancer data base. Cancer 2008; 113: 78-83
- Lee CT, Katz J, Fearn PA, Russo P.?Mode of presentation of renal cell carcinoma provides prognostic information. Urol Oncol 2002; 7: 135-40
- Eble JN, Sauter G, Epstein JI, Sesterhenn IA. editors?In: Pathology and Genetics of Tumors of the Urinary System and Male Genital Organs. World Health Organization Classification of Tumors. Lyons: IARC Press 2004; p: 7
- Fuhrman SA, Lasky LC, Limas C.?Prognostic significance of morphologic parameters in renal cell carcinoma. Am J Surg Pathol 1982; 6: 655-63
- Kanamaru H, Sasaki M, Miwa Y, Akino H, Okada K.?Prognostic value of sarcomatoid histology and volume-weighted mean nuclear volume in renal cell carcinoma. BJU Int 1999; 83: 222-6
- Srigley JR, Delahunt B.?Uncommon and recently described renal carcinomas. Mod Pathol 2009; 22 Suppl 2: S2-23
- Lam JS, Klatte T, Breda A.?Staging of renal cell carcinoma: Current concepts. Indian J Urol 2009; 25: 446-54
- Takashi M, Nakano Y, Sakata T, Miyake K, Hamajima N.?Multivariate evaluation of prognostic determinants for renal cell carcinoma. Urol Int 1993; 50: 6-12
- Wang Y, Huang C, Wu Y, Gao G, Xin Y, Lin Z.?et al.?Multivariate analysis of prognostic factors in renal cell carcinoma. Zhonghua Wai Ke Za Zhi 2000; 38: 442-4
- Mootha RK, Butler R, Laucirica R, Scardino PT, Lerner SP.?Renal cell carcinoma with an infrarenal vena caval tumor thrombus. Urology 1999; 54: 561
- Wagner B, Patard JJ, M?jean A, Bensalah K, Verhoest G, Zigeuner R.?et al.?Prognostic value of renal vein and inferior vena cava involvement in renal cell carcinoma. Eur Urol 2009; 55: 452-9


 PDF
PDF  Views
Views  Share
Share

