Report of chronic myeloid leukemia from All India Institute of Medical Sciences, 1990-2010
CC BY-NC-ND 4.0 · Indian J Med Paediatr Oncol 2013; 34(03): 159-163
DOI: DOI: 10.4103/0971-5851.123712
Abstract
All India Institute of Medical Sciences, New Delhi is an Apex Institute and caters to more than 1.5 million out-patients and 80,000 in-patients every year. In this study, we have presented data of patients over a period of 20 years. This encompasses our initial experience with hydroxyurea, then interferon alone or with cytarabine and finally imatinib. We have presented data of 525 patients treated on imatinib alone. Imatinib dose was increased in 56 (10.6%) patients and regain of complete hematological response was seen in 26 patients. A total of 14 patients were transplanted for different indications of chronic myeloid leukemia and out of which 12 patients are doing well, while two died due to Grade IV gut graft-versus-host disease.
Publication History
Article published online:
19 July 2021
© 2013. Indian Society of Medical and Paediatric Oncology. This is an open access article published by Thieme under the terms of the Creative Commons Attribution-NonDerivative-NonCommercial-License, permitting copying and reproduction so long as the original work is given appropriate credit. Contents may not be used for commercial purposes, or adapted, remixed, transformed or built upon. (https://creativecommons.org/licenses/by-nc-nd/4.0/.)
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India
Abstract
All India Institute of Medical Sciences is an Apex Institute and caters more than 1.5 million out-patients and 80,000 in-patients every year. In this study, they have presented interesting results of patients treated with interferon, interferon plus cytarabine and then with imatinib plus cytarabine. They have presented data of 525 patients treated on imatinib alone. Imatinib dose was increased in 56 (10.6%) patients and regain of complete hematological response was seen in 26 patients. A total of 14 patients were transplanted for different indications of chronic myeloid leukemia and out of which 12 patients are doing well, while two died due to Grade IV gut graft-versus-host disease.
INTRODUCTION
Chronic myeloid leukemia (CML) has been one of the few success stories of cancer therapy today.[1,2] One of the authors (PM) saw the arrival of this drug as a research officer and we have seen it over the time grow into one of the wonder drugs of cancer therapy. We have now attended endless debates and programs on the drug and its newer rival. We have seen extravagant optimism at its launch, heard the cynics grow louder sometime later (resistance! just you wait and see) and finally a more informed acceptance of the drug and its limitations. Today, there is no denying its place in medical history. Therefore, it is good that we share our own experiences with this drug.
OUR EXPERIENCE WITH CML
We have a separate out-patients department (OPD) register for patients with CML. We have on record 1105 patients as on June 2010. This includes all new as well as older patients on follow-up. The max foundation patient assistance program alone has supported 987 patients of whom 639 (64%) patients are in active follow-up with the foundation. Most of the other patients are on generic brands supported by their employers or their own funds. The max foundation offers 11 months of free treatment for some patients. As per our records only one patient has availed of this scheme. All patients still find it economically viable to take generic brands rather than buy 1 month supply of Glivec. We have not studied all 1105 patients together in detail. Our data is still piecemeal. Thus, we have initial data on patients during the time when almost all patients were on hydroxyurea. Then we have some data on our experience with interferon. The interferon study stopped accrual with the advent of generic imatinib, which was considerably cheaper than Glivec. We supplanted imatinib in the interferon trial initially and have some data on imatinib with cytarabine. We took the invitation by TMH to present our data as an incentive to put our data in order. Over the last few months we collected basic data, which we could analyze quickly in time for the meeting. We could gather data on 525 patients (restricting ourselves to only those who had at least 6 months of follow-up and excluded repeat follow-ups during this time). In addition, we have also presented our experience with children, CML in pregnancy and transplant.
THE INTERFERON PERIOD
Some of the readers might remember the interferon trial funded by Fulford, which studied interferon alpha for CML.[3] At the time imatinib was in phase II trials. My job was to recruit patients for the project, randomize them to two arms — one receiving interferon alone and the other in combination with cytarabine. Cytogenetic studies at the time were performed by a PhD student in anatomy. Prof. Choudhry had analyzed data on patients presenting with CML prior to starting the interferon project. He had compared the presenting features of patients presenting with CML as compared with a North American population [Table 1].
Table 1
Patient characteristics of patients presenting with CML in comparison with West data
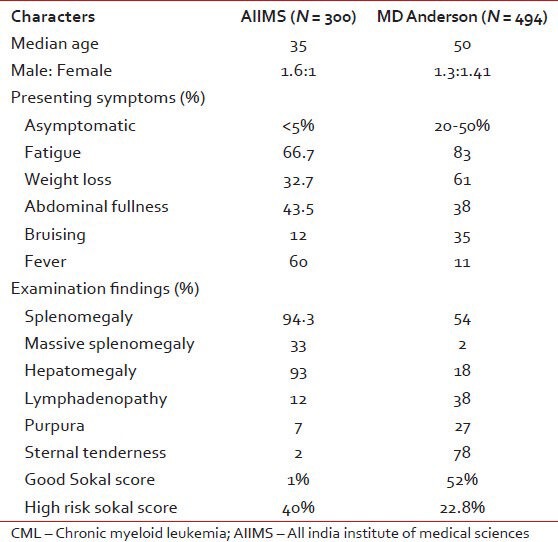
We had complete data on 73 patients enrolled in the interferon study. Baseline data and results are tabulated in Tables Tables22 and and33.
Table 2
Patient characteristics in the interferon trial
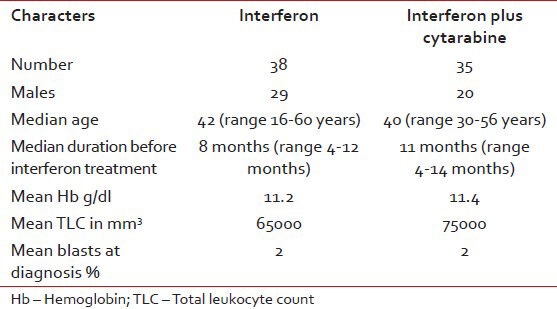
Table 3
Treatment results
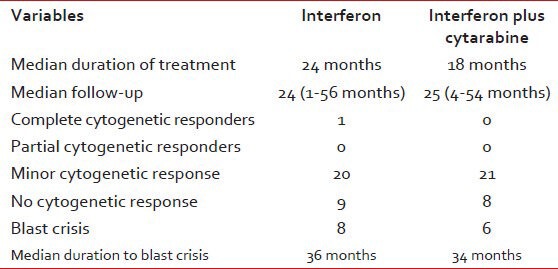
There was no significant difference in toxicities between the two arms. The common side-effects were fever (24%), body ache (40%) and loss of appetite (34%), neutropenia (30%). However, all patients could not tolerate doses greater than 3 min thrice a week. Two patients developed psychosis and two patients had renal failure requiring dialysis. There was no added toxicity to the addition of cytarabine. However, the cytogenetic responses in this group were very disappointing.
At around this time, Glivec was introduced in the market. It was and still is beyond the reach of most patients in India. With the advent of generic forms, interferon quickly fell out of fashion. The interferon project was fast losing patients who crossed over to the generic forms. We then continued our study, this time substituting imatinib for interferon.
THE GLIVEC YEARS
Our job of enrolling patients was aided in no small measure by the Max foundation, which freely provided imatinib to almost all patients excepting those covered by the employer's (or government service) medical cover. Professor Saxena had already established the molecular laboratory with Dr Sudha Sazawal. The PhD student had completed her theses by this time and there was no one else doing cytogenetics in the hospital (perhaps someone in medical oncology for a while). Therefore, we decided to make do with what we had and decided to monitor these patients with quantitative polymerase chain reaction (PCR). The test was not standardized at the time and we kept samples at 3 monthly intervals. We enrolled 85 patients in this study.
Only those patients in chronic phase with no coexisting morbidities or pregnancy were included in the study. All patients were started on imatinib within 6 months of diagnosis. Hydroxyurea was the only treatment allowed prior to starting imatinib for inclusion in the study. Imatinib was started in 40 patients at the standard recommended dose of 400 mg/day. Cytarabine was added in 45 patients at a dose of 10 mg/m2 for 10 days every month, after 3 months of imatinib. We had problems with quantifying the molecular response at the time and out of the 85 patients enrolled in the study, we were able to perform the real time PCR for BCR-ABL at baseline and follow-up in 56 patients. 36 patients had received gleevec and 20 patients had received cytarabine in addition to gleevec. The results are tabulated in Tables Tables44–7.
Table 4
Baseline data in the AIIMS imatinib study
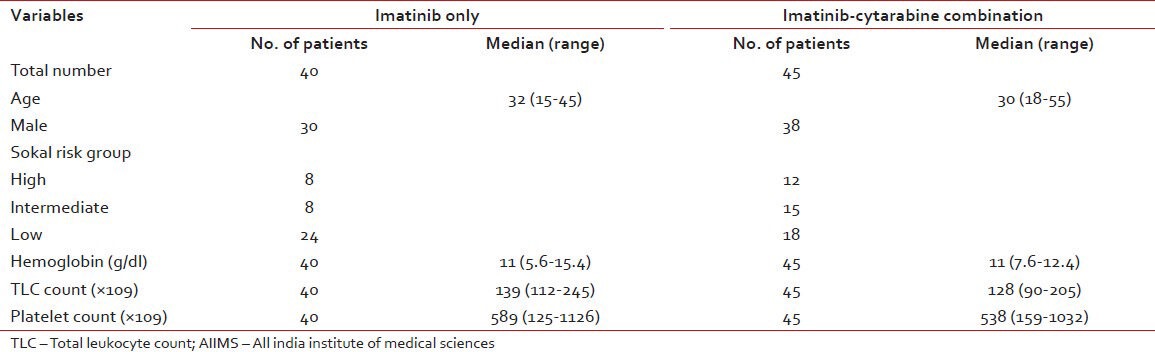
Table 7
Real time PCR results
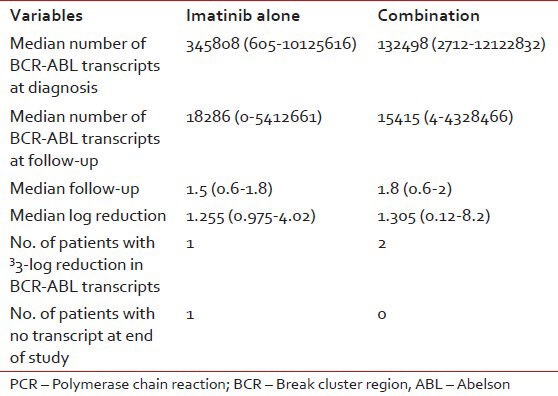
This initial study showed that all patients achieved complete hematological response within a period of 1-3 months after starting imatinib. None of these patients lost their hematological responses at the end of the 3 years study period. We had several problems during the molecular analyses. Our laboratory was used to getting the values in terms of transcript levels and we tried to use these values to get the log reductions and then extrapolate them to IRIS norms. This was one of the major limitations of our study and could be the reason for the seemingly poor molecular response. All patients analyzed were newly diagnosed patients who received Glivec soon after diagnosis. Subsequently the laboratory got around to giving results in the more commonly used BCR-ABL/ABL ratio format. Even so we felt that the best way about would be to standardize our results with one of the IRIS laboratories. We now have an understanding with the laboratory at Adelaide, who has agreed to help us.
One of the major blocks in getting patients to do cytogenetic/molecular response analyses is that most of our patients at All India Institute of Medical Sciences (AIIMS) do not have any option beyond imatinib owing to financial reasons. Those who can afford and are motivated do go in for regular analyses at 6 monthly intervals and this has helped us to increase the dose of imatinib for those whose BCR-ABL levels showed a rising trend.
OUR CURRENT DATA ON CML
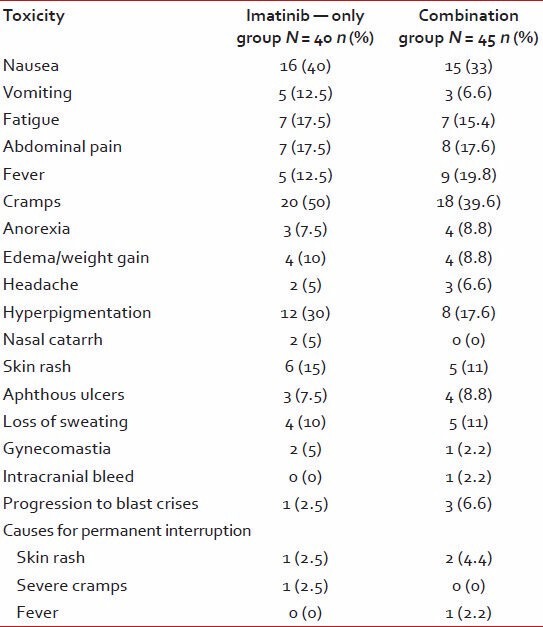
Some of the 525 patients who are currently been analyzed have been included in the previous data already presented above. Therefore, the baseline data is not very different from those already presented in Tables Tables11 and and44 [patients enrolled in the interferon study had a higher median age in the and lower baseline total leukocyte count levels -Table 2]. We did not again analyze the hematological and non-hematological toxicities of imatinib, which were already analyzed in the imatinib study [Tables [Tables55 and and6].6]. Thus, this data is more of a follow-up data of those patients who are currently attending the OPD.
Table 5
Hematological toxicities
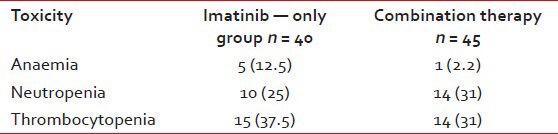
Table 6
Non-hematological toxicities
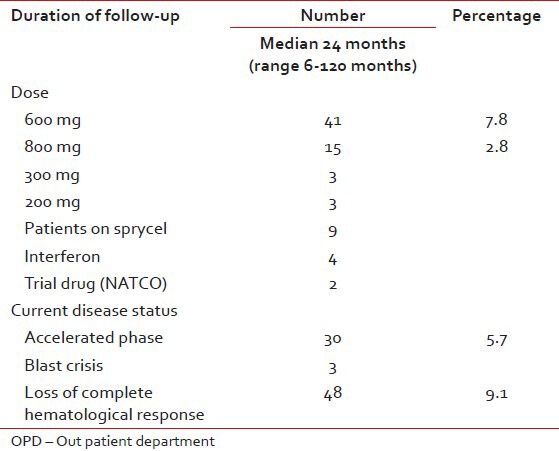
We do not currently have data on those patients who died as this data is OPD based. Once we have compiled the OPD data, we will then go to those files, which are missing from the list and try and analyze the reasons for dropout. Once these data are available then disease status at follow-up would be expected to be different than what is currently seen from Table 8.
Table 8
Follow-up data on 525 patients currently on follow-up in hematology OPD as on June 2010
We did not actively persuade any patient to undergo analysis to look for molecular or cytogenetic response considering the fact that most of these patients do not have recourse to any other treatment option and molecular analyses even at AIIMS costs Rs. 3,000. We did explain the importance of such this test at the beginning. Of these 525 patients 123 patients underwent molecular or cytogenetic testing at least once during follow-up [Table 9]. We generally advised such tests after at least 6-12 months of follow-up.
Table 9
Molecular/cytogenetic analyses for 123 patients (out of 525 patients)
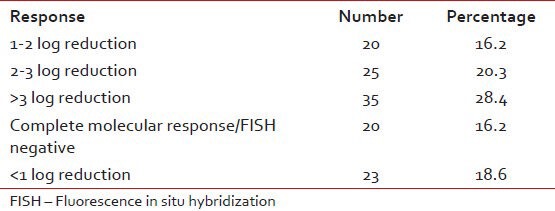
Imatinib dose was increased in 56 patients. The reason for dose increase was a loss in hematological response in 25, inadequate molecular response in 18 and accelerated phase in 13 patients. Of these 56 patients an increase in dose helped regain complete hematological response in 26 patients.
SPECIAL GROUPS: CML IN CHILDREN AND PREGNANCY
We analyzed 41 cases of adult CML in childhood, seen over a period of 10 years from 1994 to 2004 in our department. The disease was usually seen in children older than 12 years (87.8%) (age range: 2-16 years). Males predominated in this group (27 vs. 14). Most of the children presented in the chronic phase (85%). Using the Sokal score based on age, spleen, platelets and blast count 80%, 14.6% and 29.2% were in the low, intermediate and high-risk groups respectively. Our patients usually presented with fever, abdominal mass/pain and anorexia. Though, hepatosplenomegaly was common, lymphadenopathy was rare (one patient). The median survival was 13 months (range: 2-144 months). We had one interesting case of Philadelphia positive thrombocytosis in a girl, 9 years old at the time who required both hydroxyurea and imatinib initially to correct her counts. Imatinib was able to bring her BCR-ABL levels down, but somehow did not affect her platelet counts. We published this case in the European journal of hematology. She is 6 years into follow-up and is on imatinib alone. Her BCR-ABL levels are undetectable. For those who are interested, she does not have a matched sibling.
One of the authors (PM) had presented our data on CML in pregnancy at the last meeting hosted by TMH. We now have 10 patients who became pregnant during the course of their disease. All became pregnant while on imatinib. We had followed the manufacturer advice and asked them to stop the medication. Only three complied with the instruction. The rest had uneventful outcomes, except for one whose child had a meningocele.
TOLERABILITY AND PRIMARY RESISTANCE
Imatinib has largely been a safe and well tolerated drug. We had one patient who attempted to commit suicide by consuming a large number of Glivec tablets, but failed miserably. We have three patients on record who did not tolerate even 200 mg of imatinib and is now on interferon. Three other patients are on 300 mg of imatinib. Of these six patients on a suboptimal dose only one is in complete hematological response. We have had primary resistance in two patients till date. We found a matched sibling for one of them and he was transplanted. He had a T315I mutation to start with. After transplant his BCR-ABL levels showed a rising trend after 3 months. He was planned for donor lymphocyte infusion (DLI) but has extensive skin graft-versus-host disease (GVHD) and also liver involvement. He was negative for the T315I mutation after transplant and he is currently on Dasatinib. We do not currently have the exact figures for secondary resistance, but they do not appear to be greater than what has been reported.
TRANSPLANT IN CML
We have transplanted 14 patients with CML since 2004. Eight patients were in accelerated or blast phase at the time of transplant. One patient was primary refractory to imatinib (described before). Six patients were in chronic phase showing loss of molecular response and progressively rising transcript levels even after increasing the dose of imatinib in three patients. Three of these six patients elected to have a transplant instead of increasing their dose. We lost two of these patients, both to grade IV gut GVHD. Two of these 14 patients were children one of whom, a 3-year-old was initially managed for a lymphoid blast crisis. Four of these 14 patients lost their molecular response and two opted for imatinib alone and subsequently went into complete molecular response. One of the other two patients is the primary refractory patient described above and we are planning a DLI for the latest patient who has lost molecular response.
OUR IMMEDIATE GOALS
We hope to standardize our laboratory results for molecular monitoring by the end of the year. We are also currently part of a drug trial sponsored by NATCO who have developed an indigenous tyrosine kinase inhibitor. We are steadily building up some data on patients on Dasatinib. All patients on dasatinib are currently those who are covered by the central government health scheme. The financial implications of the newer tyrosine kinase inhibitors have forced us to look anew at Interferon in a few patients.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.


 PDF
PDF  Views
Views  Share
Share

