Report of patients with chronic myeloid leukemia Kidwai Memorial Institute of Oncology, Bangalore over 15 years
CC BY-NC-ND 4.0 · Indian J Med Paediatr Oncol 2013; 34(03): 196-198
DOI: DOI: 10.4103/0971-5851.123736
Abstract
Kidwai Memorial Institute of Bangalore is one of the most comprehensive and major regional cancer center. It was established in 1974 and caters population not only from Karnataka, but also from Tamil Nadu, Andhra Pradesh. They presented the data of 540 patients out of which 95% patients were in chronic phase. Complete hematological response was seen in 98.45 of patients. Overall survival at 5 years was 86.5%.101 patients has suboptimal response were considered for and underwent mutational analysis, out of which 27 patients showed various mutations and are mentioned elaborately in the article.
Publication History
Article published online:
19 July 2021
© 2013. Indian Society of Medical and Paediatric Oncology. This is an open access article published by Thieme under the terms of the Creative Commons Attribution-NonDerivative-NonCommercial-License, permitting copying and reproduction so long as the original work is given appropriate credit. Contents may not be used for commercial purposes, or adapted, remixed, transformed or built upon. (https://creativecommons.org/licenses/by-nc-nd/4.0/.)
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India
Abstract
Kidwai Memorial Institute of Bangalore is one of the most comprehensive and major regional cancer center. It was established in 1974 and caters population not only from Karnataka, but also from Tamil Nadu, Andhra Pradesh. They presented the data of 540 patients out of which 95% patients were in chronic phase. Complete hematological response was seen in 98.45 of patients. Overall survival at 5 years was 86.5%.101 patients has suboptimal response were considered for and underwent mutational analysis, out of which 27 patients showed various mutations and are mentioned elaborately in the article.
DISCUSSION
In our cohort of patients treated over a period of 15 years, almost 80% were still on imatinib. Among the patients who did not show adequate response or resistance to Imatinib underwent mutation analysis and only 27% had IRMA +ve mutations, rest 73% did not have any recognizable mutations. Our data reemphasizes that there are many other mechanisms involved for imatinib resistance and early recognistion and early intervention of such patients can improve the overall outcome.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
REFERENCES
INTRODUCTION
Imatinib has revolutionized the treatment of chronic myeloid leukemia (CML) globally. Due to Imatinib the basic defect in the disease has been targeted and more and more survival advantage is being achieved.[1]
We at Kidwai Memorial Institute of Oncology, Bangalore are using this targeted therapy from almost 15 years.
PATIENTS AND METHODS
We investigated patients with baseline complete blood count, peripheral smear, biochemistry including random blood sugar, renal function test and liver function tests. All patients underwent bone marrow (BM) aspiration at diagnosis as well as biopsy and cytogenetics. Patients also underwent baseline molecular testing of quantitative BCR-ABL transcript estimation.
Patients were started on Imatinib. The doses of Imatinib were 400 mg OD in chronic phase (CP) and 600 mg OD in patients who presented in accelerated and blast phase. The patients who were on Imatinib at our center, underwent testing for hematological response every month, cytogenetics every 6 month and molecular response (MolR) every year. However, this reassessment could not be strictly followed due to various reasons and financial constraints being the most common reason.
Response definitions and treatment recommendations
These were based on the hematologic response (HR), cytogenetic response (CyR) and MolR was as follows.
Criteria for HR
Complete HR (CHR) white blood cell < 10 × 109/L, Basophils < 5%, No myelocytes, promyelocytes, myeloblasts in the differential, Platelet count < 450 × 109/L, Spleen nonpalpable.
Criteria for CyR
Complete CyR (CCyR) - No Ph + metaphases
Partial CyR (PCyR) - 1% to 35% Ph + metaphases
Minor CyR - 36% to 65% Ph + metaphases
Minimal CyR - 66% to 95% Ph + metaphases
None CyR >95% Ph + metaphases.
Criteria for MolR
Complete molecular response — Undetectable BCR-ABL messenger ribonucleic acid transcript by real time quantitative and/or nested polymerase chain reaction, major molecular response (MMolR) - >3 log reduction in BCR-ABL transcript.
Patients who did not achieve either hematologic or cytogenetic or molecular milestones or had a loss of response were studied for mutation status also.
RESULTS
Over 15 years we have treated 540 Patients at Kidwai Memorial Institute of Oncology with Imatinib, Out of these 540 patients, 448 Patient are still on Imatinib.
Median age at presentation was 38.4 years 2 patients presented in blast crisis, 3 in accelerated phase (AP) and 67 in CP. Male to Female ratio was 1.7:1.0. Most common complaint was pain abdomen and swelling. The abdomen swelling was due to splenomegaly. Average spleen size was 11.32 cm (2-20 cm).
Mean hemoglobin at presentation was 9.057 mg/dl. Mean peripheral blasts were 4% with average BM blasts – 4.38%.
On classifying the patients into CP AP and blastic phase (BP), most of the patients i.e., 95% were in CP, among the rest of the patients 33% were in AP and 1.10% in BP.
We also calculated the sokal risk score of the patient and most of the patients (63.33%) were intermediate score group.
At the end of 3 months, 7 (1.5%) patients had not achieved complete hematological remission and rest 441 (98.4%) patients had achieved CHR.
At the end of 6 month, CHR was seen in the same number of patients. CCyR was achieved in 135 (30.13%) and 10 (2.23%) patients did not show any CyR, these patients were considered as a failure and underwent Imatinib resistant mutation analysis IRMA. Major Molecular Response MMolR was seen in 85 (19%) of patients.
At the end of 12 months, 98.2% patients were in CHR. This was due to the loss of hematological response in one patient at 10 months. This patient was considered as a failure and also underwent IRMA. 298 (66.52%) patients were in CCyR, but 28 (6.25%) patients had not achieved PCyR and hence these patients also underwent IRMA. 149 (33.3%) patients were in MMR.
At the end of 18 months, 98.2% patients were in CHR. 351 (78.35%) patients were in CCR and 165 (37%) patients were in MMR. So, 97 patients were not in CCR at the end of 18 months. Although, these patients were asked for IRMA most of the patients did not undergo the test because of financial constraints.
Percentage of patients showing CCR and MMR showed a continuous increase with time and is still increasing.
At an average follow-up of 68.4 months 448 (82.96%) patients are still on Imatinib. Among, rest of 92 patients, 21 (3.89%) patients discontinued therapy because of intolerance. Rest of the patients were either lost to follow-up or died.
Overall survival at this time is 86.85%. 322 (71.88%) patients were in complete cytogenetic remission.138 (30.8%) patients were in MMR. 47 (10.49%) patients were in complete molecular response.
Side-effects
Most common side-effects observed were facial rash (95.2%), myelosuppression (91%), GI symptoms (88.6%), myalgias (65.4%) and arthralgias. However, these symptoms were manageable with symptomatic treatment and modification of dose in most of the patients. Only 21 (3.89%) patients had to discontinue therapy because of major side-effects even after reduction of dose. Most of these patients were discontinued therapy because of myelosuppression even at 200 mg OD dosing.
Patients who did not achieve major milestones on follow-up or had shown a loss of response hematological/cytogenetic/molecules underwent testing for IRMA. 7 patients did not achieve CHR at 3 months. Hence, these were the candidates of IRMA. 1 patient had a loss of HR at 10 months. 42 patients did not achieve cytogenetic milestones. 28 patients had a loss of CyR at the end of 24 months. 15 patients did not achieve MMolR at 24 months and 8 had a loss of MolR even after achieving it.
Mutation analysis
A total of 101 patients underwent IRMA out of which 73% did not show any mutation, but the rest of the patients had one or other mutation.[2,3]
We did Imatinib resistance analysis in patients who did not achieve major milestones or had a loss of response.
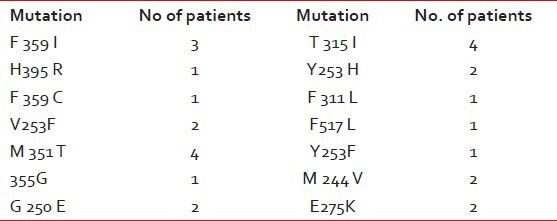
IRMA was done in 101 patients. Out of 101 patients, 74 patients did not have any mutations. Rest of the 27 patients had IRMA +ve with following mutations as shown in Table 1.
Table 1
Showing the various mutations seen in 27 patients
DISCUSSION
In our cohort of patients treated over a period of 15 years, almost 80% were still on imatinib. Among the patients who did not show adequate response or resistance to Imatinib underwent mutation analysis and only 27% had IRMA +ve mutations, rest 73% did not have any recognizable mutations. Our data reemphasizes that there are many other mechanisms involved for imatinib resistance and early recognistion and early intervention of such patients can improve the overall outcome.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.


 PDF
PDF  Views
Views  Share
Share

