Retrospective analysis of the clinical and demographic variables on the outcomes after second-line treatment in advanced non-small cell lung cancer
CC BY-NC-ND 4.0 · Indian J Med Paediatr Oncol 2013; 34(04): 274-279
DOI: DOI: 10.4103/0971-5851.125244
Abstract
Background: Platinum based doublets chemotherapy are the standard of care for metastatic or advanced non-small cell lung carcinoma. This leads to modest survival advantage and improve quality-of-life. However, patients with advanced or metastatic disease eventually present disease progression and needs second-line systemic therapy in a selected group of patients or other supportive measures. There is very little knowledge available from the literature about the prognostic variables in patients, who receive second-line therapy. Materials and Methods: We retrospectively reviewed 329 patients received second-line treatment from July 2007 to September 2011 in the Department of Radiation Oncology, Burdwan Medical College and Hospital. For statistical analysis, 12 potential prognostic variables included. Univariate and multivariate regression analysis carried out to identify the prognostic variables associated with survival. Results: The results of univariate analysis for overall survival (OS) and survival after second-line therapy identified to have prognostic significance: Age, sex, performance status, smoking history, serum lactate dehydrogenase, histopathology, first-line chemotherapy and its response and second-line therapy except the stage at diagnosis and site of failure after first-line therapy. The multivariate Cox regression analysis has shown that only performance and second-line therapy were independent prognostic variables for survival after second-line treatment and above these prognostic factors; age, smoking status and progression free survival also for OS. Conclusion: The performance status has shown consistent result as a prognostic factor in univariate and multivariate analysis for OS and survival after second-line therapy. These findings may also facilitate pretreatment prediction of survival and be used for selecting patients for the correct choice of cytotoxic therapy.
Keywords
Non-small cell lung carcinoma - overall survival - prognostic variables - second-line chemotherapyPublication History
Article published online:
19 July 2021
© 2013. Indian Society of Medical and Paediatric Oncology. This is an open access article published by Thieme under the terms of the Creative Commons Attribution-NonDerivative-NonCommercial-License, permitting copying and reproduction so long as the original work is given appropriate credit. Contents may not be used for commercial purposes, or adapted, remixed, transformed or built upon. (https://creativecommons.org/licenses/by-nc-nd/4.0/.)
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India
Abstract
Background:
Platinum based doublets chemotherapy are the standard of care for metastatic or advanced non-small cell lung carcinoma. This leads to modest survival advantage and improve quality-of-life. However, patients with advanced or metastatic disease eventually present disease progression and needs second-line systemic therapy in a selected group of patients or other supportive measures. There is very little knowledge available from the literature about the prognostic variables in patients, who receive second-line therapy.
Materials and Methods:
We retrospectively reviewed 329 patients received second-line treatment from July 2007 to September 2011 in the Department of Radiation Oncology, Burdwan Medical College and Hospital. For statistical analysis, 12 potential prognostic variables included. Univariate and multivariate regression analysis carried out to identify the prognostic variables associated with survival.
Results:
The results of univariate analysis for overall survival (OS) and survival after second-line therapy identified to have prognostic significance: Age, sex, performance status, smoking history, serum lactate dehydrogenase, histopathology, first-line chemotherapy and its response and second-line therapy except the stage at diagnosis and site of failure after first-line therapy. The multivariate Cox regression analysis has shown that only performance and second-line therapy were independent prognostic variables for survival after second-line treatment and above these prognostic factors; age, smoking status and progression free survival also for OS.
Conclusion:
The performance status has shown consistent result as a prognostic factor in univariate and multivariate analysis for OS and survival after second-line therapy. These findings may also facilitate pretreatment prediction of survival and be used for selecting patients for the correct choice of cytotoxic therapy.
INTRODUCTION
Metastatic or loco-regionally advanced non-small cell lung carcinoma (NSCLC) is one of the leading causes of death attacked with cancer in India and though treatment outcomes in advanced disease remain modest, with the paradigm shift in the approach to treatments. They are steadily improving. Customized treatment based on the tumor histology and genetic profiles have become the standard of care.
Systemic chemotherapy with the platinum based doublets is the standard of care in metastatic or advanced NSCLC.[1,2,3] This leads to modest survival advantage and improve quality-of-life.
However, patients with advanced or metastatic disease eventually present disease progression and needs second-line systemic therapy in a selected group of patients or other supportive measures.
Second-line chemotherapy with docetaxel had shown to improve overall survival (OS) by means of 3 months and to delay the deterioration of quality-of-life in patients with good performance status.[4]
Pemetrexed is another anti-folate approved for use in non-squamous histology NSCLC as second-line chemotherapy and has emerged as a preferred option in this sub-group.[5,6]
The NCIC trial BR21 had shown that the benefit of erlotinib after of first-line chemotherapy in NSCLC. Treatment with erlotinib improves progression free survival (PFS) (hazard ratio: 0.61, P=0.001) and then erlotinib approved for the treatment of advanced NSCLC after the failure of upfront chemotherapy.[7]
Interest, phase III high-powered randomized study and the patients randomized to receive gefitinib or docetaxel as second-line therapy.[8] The analysis showed that the objective response and survival were similar in the two groups and consequently approved as second-line therapy in NSCLC.
In spite of the clinical benefit of the second-line treatment, it is often to observe the toxic side effect. There are reliable predictors of objective response, those who received first-line chemotherapy, but very little knowledge available from literature about the prognostic variables in patients, who receive second-line therapy. There is a modest survival advantage in second-line therapy, at the same time leaves toxic side-effect. We need to find out robust clinical and demographic predictive and prognostic factors to guide the second-line therapy in NSCLC after disease progression.
The objective of this study was to identify the clinical or demographic predictive factors for survival of advanced or metastatic NSCLC who received second-line therapy.
MATERIALS AND METHODS
Patient population
We retrospectively reviewed 329 patients received second-line treatment from July 2007 to September 2011 in the Department of Radiation Oncology, Burdwan Medical College and Hospital.
They met the following inclusion criteria;
- Histological or cytological proof of NSCLC
- Stage III-IV (brain metastasis excluded)
- Received first-line platinum based doublet chemotherapy
- They had measurable disease as defined by WHO criteria
- They were 18 years or older in age.
Factors analyzed
A total of 12 potential prognostic variables were chosen as the basis of previously published clinical trials. The variables were categorized as
- Age (60 years vs. >60 years)
- Sex (male/female)
- Performance status (ECOG 0-1/2-3)
- Smoking status (never smoker/smoker or formerly smoker)
- Serum lactate dehydrogenase (normal/elevated)
- Histology (adenocarcinoma/non-adenocarcinoma)
- Stage at diagnosis (Stage IIIA and IIIB or Stage IV)
- First-line chemotherapy (platinum based doublet, etoposide vs. paclitaxel or gemcitabine or pemetrexed)
- Response to first-line chemotherapy (clinical disease control or progressive disease)
- PFS after first-line chemotherapy (6 months/>6 months)
- Site of failure (intrathoracic vs. intra-thoracic and distant/distant/brain metastasis)
- Second-line chemotherapy (base supportive care vs. docetaxel/pemetrexed/erlotinib/gefitinib)
Statistical analysis
All of the analysis performed using the SPSS Statistical Software Program Package (SPSS Version 16, SPSS Inc., Chicago). The difference of the clinical characteristic between the groups was analyzed by Chi-square test and Student t-test. OS calculated from the first cycle of chemotherapy to the date of death from any cause or the date of last follow-up.
Survival after second-line chemotherapy calculated from the 1st day of starting second-line therapy to the date of death from any cause or date of last follow-up. PFS defined as the period from the first day of chemotherapy until the progression of disease or death. PFS and OS estimated using the Kaplan-Meier method. The multivariate analysis by Cox regression model used to determine statistical significant variables. The difference was assumed to be significant when P=value < 0.05.
RESULTS
Patient characteristics
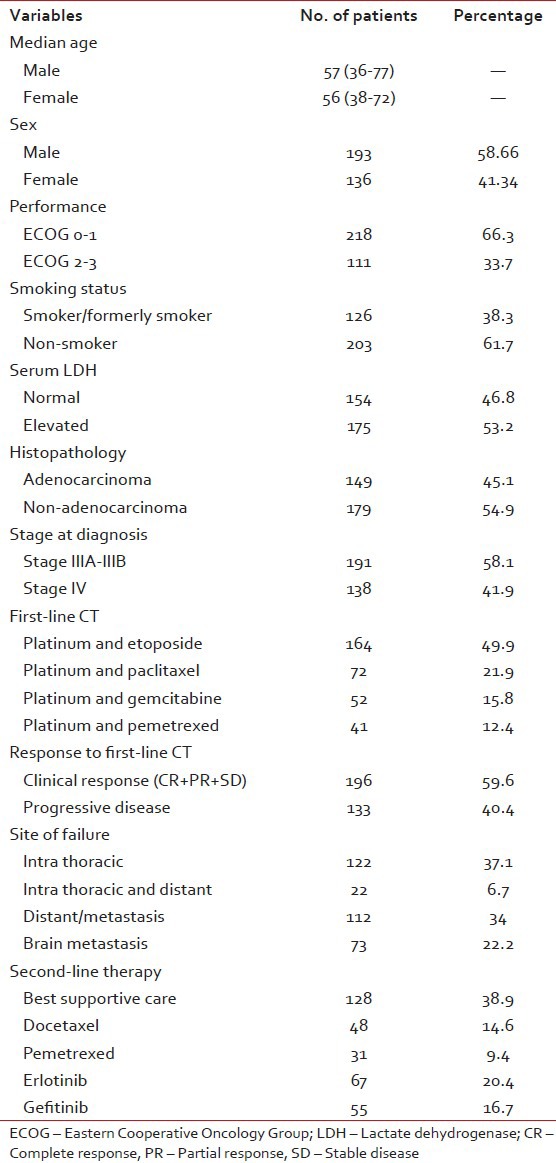
Between July 2007 and September 2011, 329 patients received second-line systemic therapy or best supportive care (BSC) after failing platinum based doublet chemotherapy. The median age of the female and male patients was 56 and 57 respectively. Patients with good (ECOG 0-1) and poor performance (ECOG 2-3) status was 66.3% and 33.7% respectively. Less than half (42%) of the patients were presented with metastatic disease.
Squamous is the most common (56%) histological type. There was a significant association between the tumor histology and sex. Adenocarcinoma was the most common among women. The median survival after the starting of second-line treatment or BSC was 5 months. 27 patients were also received third-line system therapy [Table 1].
Table 1
Baseline characteristic of patients
Prognostic factor analysis
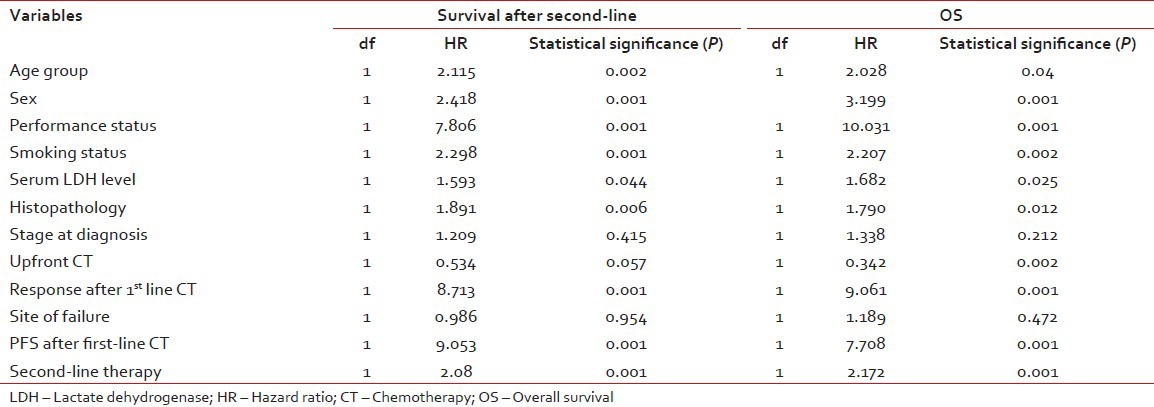
The results of univariate analysis of survival after second-line treatment and OS are summarized in Table 2. Among the 12 variables included for binary logistic analysis, all the above identified to have significant prognostic factors for survival after second-line treatment except the stage at diagnosis, 1st line chemotherapy and site of failure after 1st line chemotherapy.
Table 2
Univariate analysis of categorical variables
Multivariate analysis by Cox regression model included the prognostic significant variables from the univariate analysis.
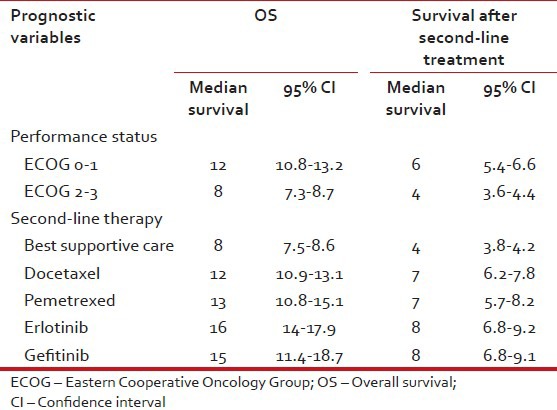
Multivariate analysis by Cox regression model has shown that performance status (PS) and second-line treatments were independent prognostic factors for survival after second-line treatment. Furthermore, performance status, age, smoking habits, PFS after 1st line therapy and second-line therapy were the variables considered an independent prognostic factor for OS [Tables [Tables33 and and4,4, Figures Figures11–4].
Table 3
Multivariate analysis of categorical variables by Cox regression model for survival after second line therapy and OS
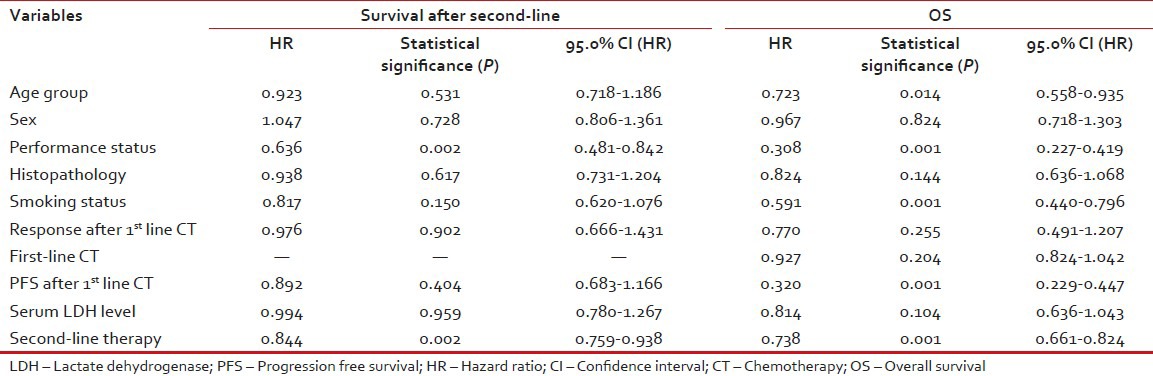
Table 4
Prognostic variables, OS and survival after second-line treatment
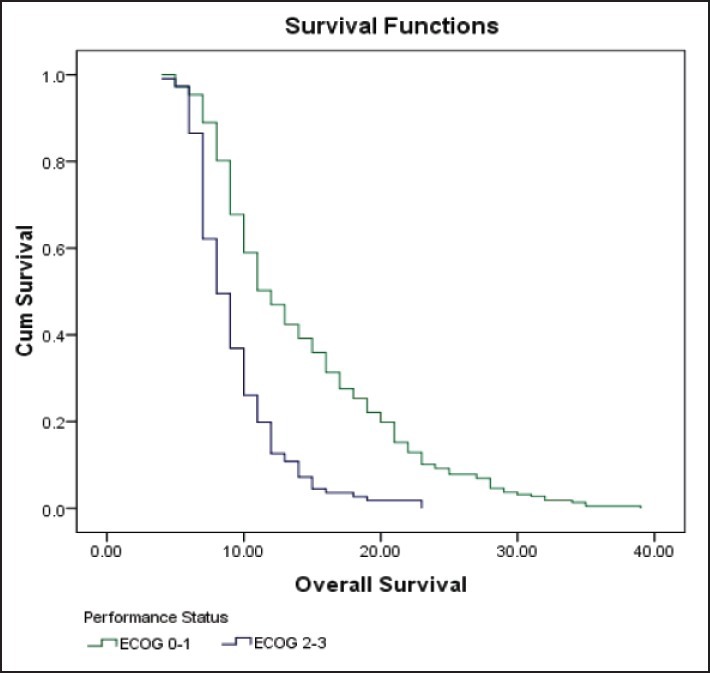
| Fig. 1 Kaplan-Meier curve estimates of overall survival in patients with ECOG 0-1 or ECOG 2-3 performance status
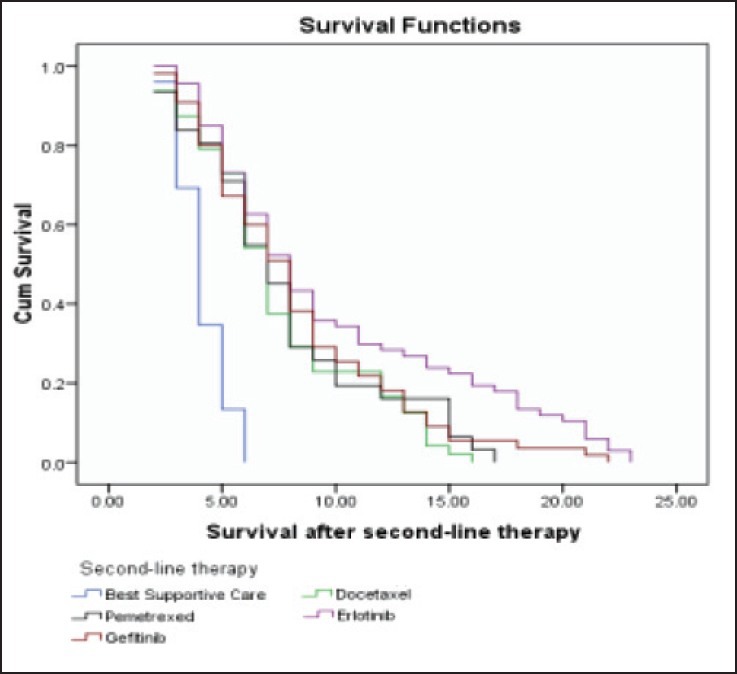
| Fig. 4 Kaplan-Meier curve estimates of survival after second line therapy in patients treated with docetaxel/pemetrexed/erlotinib/gefitinib or best supportive care
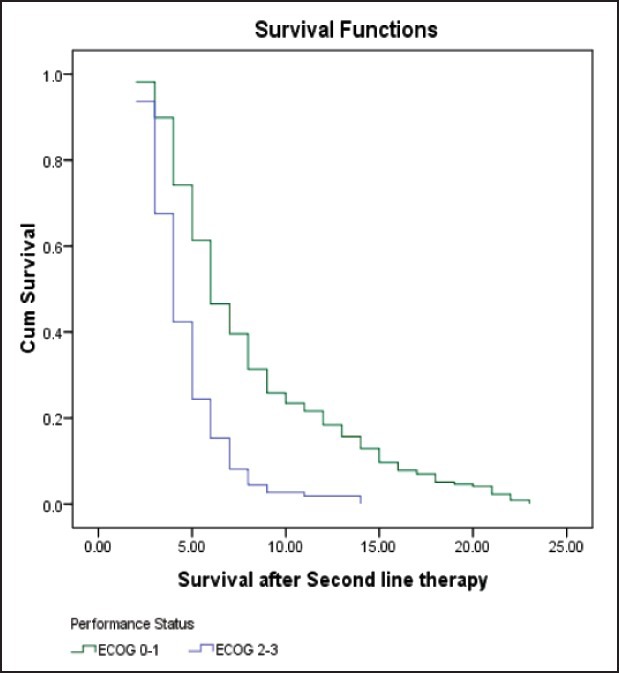
| Fig. 2 Kaplan-Meier curve estimates of survival after second-line therapy in patients with ECOG 0-1 or ECOG 2-3 performance status
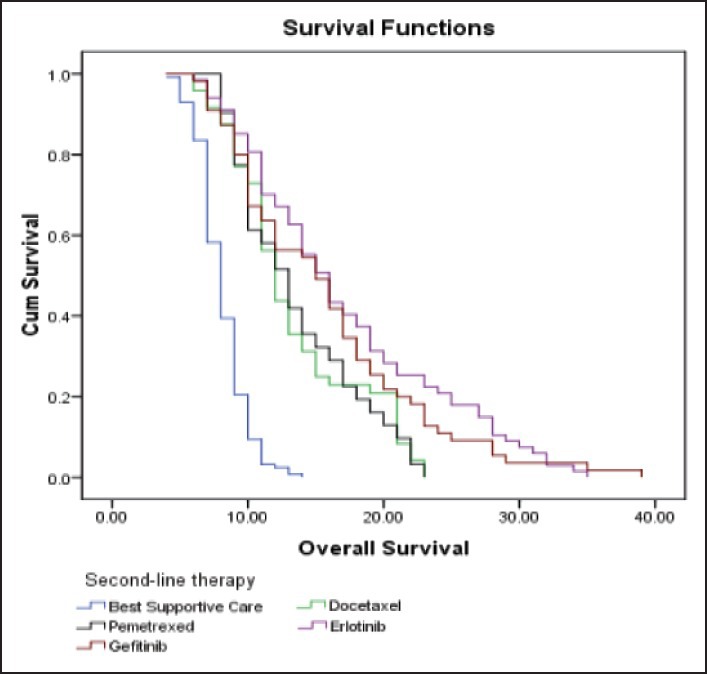
| Fig. 3 Kaplan-Meier curve estimates of overall survival in patients treated with docetaxel/pemetrexed/erlotinib/gefitinib or best supportive care
DISCUSSION
Docetaxel, Pemetrexed and TKIs (Erlotinib and Gefitinib) have significantly changed the treatment paradigm for advanced NSCLC. However, the optimal integration of first-line and second-line chemotherapy is yet to be defined.
At this time, the selection of second-line cytotoxic agent or only the BSC may depends numbers of factors including physician preferences, patients’ preferences, smoking history, smoking history and tumor histology. Give the incurable nature of the advanced NSCLC and modest survival seen after second-line setting, patient's preferences and convenience should considered when selecting the second-line agents.
Few prior studies had shown that performance status, gender, histopathology, stage at diagnosis, type of initial chemotherapy, the best response to first-line chemotherapy, PFS after upfront chemotherapy have strong predictive value in OS and OS after second-line chemotherapy in univariate analysis. However, this trend did not persist in multivariate regression analysis; the only variables have a strong correlation where the PS of patients and response to first-line chemotherapy.[9]
This retrospective study has shown the potential influence of PS and the second-line cytotoxic therapy on the outcome of the second-line treatments and the PS as well as age, smoking habits, PFS and second-line therapy had strong predictive value in OS. The outcomes were similar regardless of the response to first-line cyto-toxic therapy and did not accord with the results of.[9] This might be due in inadequate treatment with first-line chemotherapy containing etoposide and retrospective review analysis.
A poor PS accepted as a negative prognostic factor for all cancer patients.[10,11] The importance of PS also confirmed in our study and has shown poor PS had a negative effect on OS and survival after second-line therapy or BSC only. The current study demonstrated that the poor PS not only had a negative effect on OS, also did not tolerate second-line cytotoxic treatment due to toxicity.
It remains ambiguous whether stage at the time of diagnosis (IIIA, IIIB or IV) in patients receiving second-line cytotoxic agent will ensure prognostic knowledge for OS. Weiss et al., Di Maio et al., Scartozzi et al.[12,13,14] had shown significant predictive value of stage at diagnosis on the OS while Di Maio et al. and Zietemann and Duell[9,15] did not mention the prognostic value of the stage.
In our study, stage at diagnosis did not identify as a prognostic variable for OS or survival after second-line treatment. This may be due to retrospective analysis, the difference of the treatment choice and etoposide included in the first-line chemotherapy regimen. The main limitation of this study was retrospective study, we did not evaluate the molecular characteristic of tumor and first-line chemotherapy did not base upon the histopathology and small number of patients included for analysis.
CONCLUSION
The PS has shown consistent result as a prognostic factor in univariate and multivariate analysis for OS and survival after second-line systemic treatment. These findings may also facilitate pretreatment prediction of survival and be used for selecting patients for the correct choice of cyto-toxic therapy.
More prospective multicentric randomized studies are required to answer the treatment option in the second-line therapy in NSCLC or beyond. Consequently, subset and retrospective analysis might help to guide future studies and hypothesis. Two additional areas that may be worth pursuing in the data collection would be quality-of-life improvement and toxicity experiences by the patients during or after second-line therapy.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.

| Fig. 1 Kaplan-Meier curve estimates of overall survival in patients with ECOG 0-1 or ECOG 2-3 performance status

| Fig. 4 Kaplan-Meier curve estimates of survival after second line therapy in patients treated with docetaxel/pemetrexed/erlotinib/gefitinib or best supportive care

| Fig. 2 Kaplan-Meier curve estimates of survival after second-line therapy in patients with ECOG 0-1 or ECOG 2-3 performance status

| Fig. 3 Kaplan-Meier curve estimates of overall survival in patients treated with docetaxel/pemetrexed/erlotinib/gefitinib or best supportive care


 PDF
PDF  Views
Views  Share
Share

