Reversible Hypopigmentation with Pazopanib
CC BY-NC-ND 4.0 · Indian J Med Paediatr Oncol 2018; 39(04): 519-520
DOI: DOI: 10.4103/ijmpo.ijmpo_104_17
Abstract
Tablet Pazopanib known to cause Hypo pigmentation and Hyperpigmentation as per various literature reports. We report here a case of reversible hypopigmentation with Pazopanib in a patient treated for spindle cell sarcoma. Patient did not have any clinical symptoms except for cosmetic significance.
Publication History
Article published online:
17 June 2021
© 2018. Indian Society of Medical and Paediatric Oncology. This is an open access article published by Thieme under the terms of the Creative Commons Attribution-NonDerivative-NonCommercial-License, permitting copying and reproduction so long as the original work is given appropriate credit. Contents may not be used for commercial purposes, or adapted, remixed, transformed or built upon. (https://creativecommons.org/licenses/by-nc-nd/4.0/).
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India
Abstract
Tablet Pazopanib known to cause Hypo pigmentation and Hyperpigmentation as per various literature reports. We report here a case of reversible hypopigmentation with Pazopanib in a patient treated for spindle cell sarcoma. Patient did not have any clinical symptoms except for cosmetic significance.
A 25-year-old male patient was diagnosed as spindle cell sarcoma of left thigh. As per standard line of treatment, he underwent surgery, chemotherapy, and radiotherapy. After 1 year of disease-free survival, the patient presented with lung nodules. This was suggestive of metastatic disease. He completed 2nd line of chemotherapy for the same and started on maintenance therapy with tablet Pazopanib 800 mg daily from December 2015. After 3 months of this treatment, the patient developed blonde discoloration of skin and hair [Figure 1]. Starting with scalp hair, whole body skin and hair has changed to blonde color by the end of 8 months. These changes remained till the discontinuation of the tablet. There was only change in color and no clinical symptoms related to that. Patient has taken total 12 months of treatment with Pazopanib and stopped after that, because of progressive disease. Patient regained his skin and hair color back to normal, gradually over a period of 3 months after discontinuation [Figure 2] and [3].

| Figure 1:Hair and skin color changes on tablet Pazopanib
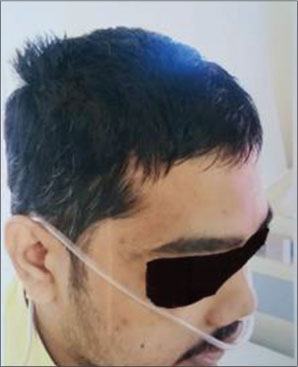
| Figure.2:Normal skin color after stopping tablet Pazopoanib
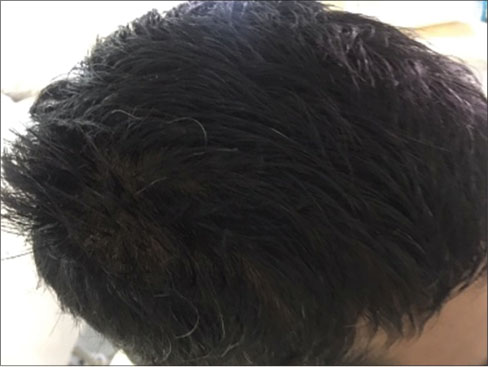
| Figure.3:Normal hair color after stopping tablet Pazopanib
Pazopanib inhibits angiogenesis and tumor growth by targeting VEGFR 1-3, PDGFR-alfa-beta, and C-kit.[1] [2] In Literature, 30%–44%-of patients have developed reversible hair pigmentation with Pazopanib.[3] [4] [5] This usually starts at 6 weeks and may go on increasing till 6 months of treatment. Both hypopigmentation[1] [4] [5] [6] and hyperpigmentation[5] have been reported but later incidence is less. C-kit and stem cell factor are required for melanocyte proliferation to melanin production during anagen phase of hair cycle.[7] Inhibition of C-kit by tyrosine kinase inhibitor (TKI) (Pazopanib) will result in failure of melanocyte differentiation and its melanin production. This hair color changes was also been reported with other TKI’s such as imatinib, dasatinib, and sunitinib.[8] Vega et al,[7] has reported two Mexican patients with synovial sarcoma, developed hypopigmentation on treatment with Pazopanib. Various phase I and phase II randomized clinical trials[9] in renal cell carcinoma,[10] nonsmall cell lung cancer,[11] and breast[12] cancer treated with Pazopanib have reported all grade hair color changes in 109 (38%), 4 (6.6%), and 14 (18%) patients, respectively. This is one of the clinical case reports to make aware about this side effect of Pazopanib to the treating oncologist.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Conflict of Interest
There are no conflicts of interest.
References
- Hurwitz HI, Dowlati A, Saini S, Savage S, Suttle AB, Gibson DM. et al. Phase I trial of pazopanib in patients with advanced cancer. Clin Cancer Res 2009; 15: 4220-7
- Hutson TE, Davis ID, Machiels JP, De Souza PL, Rottey S, Hong BF. et al. Efficacy and safety of pazopanib in patients with metastatic renal cell carcinoma. J Clin Oncol 2010; 28: 475-80
- Sternberg CN, Davis ID, Mardiak J, Szczylik C, Lee E, Wagstaff J. et al. Pazopanib in locally advanced or metastatic renal cell carcinoma: Results of a randomized phase III trial. J Clin Oncol 2010; 28: 1061-8
- Sideras K, Menefee ME, Burton JK, Erlichman C, Bible KC, Ivy SP. Profound hair and skin hypopigmentation in an African American woman treated with the multi-targeted tyrosine kinase inhibitor pazopanib. J Clin Oncol 2010; 28: e312-3
- Bible KC, Suman VJ, Molina JR, Smallridge RC, Maples WJ, Menefee ME. et al. Efficacy of pazopanib in progressive, radioiodine-refractory, metastatic differentiated thyroid cancers: Results of a phase 2 consortium study. Lancet Oncol 2010; 11: 962-72
- de Jonge MJ, Hamberg P, Verweij J, Savage S, Suttle AB, Hodge J. et al. Phase I and pharmacokinetic study of pazopanib and lapatinib combination therapy in patients with advanced solid tumors. Invest New Drugs 2013; 31: 751-9
- Teresa M, González JV, Tlahuel JL, Guerra EC. Hypopigmentation of the Skin and Hair Associated with Targeted Therapies. Journal of Cancerology 2014; 1: 67-72
- Moss KG, Toner GC, Cherrington JM, Mendel DB, Laird AD. Hair depigmentation is a biological readout for pharmacological inhibition of KIT in mice and humans. J Pharmacol Exp Ther 2003; 307: 476-80
- Elhalawani H, Heiba M, Abdel-Rahman O. Risk of distinctive hair changes associated with pazopanib in patients with renal cell carcinoma (RCC) versus patients without RCC: A comparative systematic review and meta-analysis. Clin Genitourin Cancer 2017; 15: e325-35
- Sternberg CN, Hawkins RE, Wagstaff J, Salman P, Mardiak J, Barrios CH. et al. A randomised, double-blind phase III study of pazopanib in patients with advanced and/or metastatic renal cell carcinoma: Final overall survival results and safety update. Eur J Cancer 2013; 49: 1287-96
- GlaxoSmithKline. An open-label, multicentre, randomised phase II study of pazopanib in combination with pemetrexed in first-line treatment of subjects with predominantly non-squamous cell stage IIIB wet/IV non-small cell lung cancer. Bethesda, MD: National Library of Medicine (US); 2000. Available from: https://www.clinicaltrials.gov/ct2/show/NCT 00871403?term¼NCT00871403 and rank¼1.NLM Identifier:NCT00871403. [Last accessed on 2015 Jul 29].
- Johnston SR, Gómez H, Stemmer SM, Richie M, Durante M, Pandite L. et al. A randomized and open-label trial evaluating the addition of pazopanib to lapatinib as first-line therapy in patients with HER2-positive advanced breast cancer. Breast Cancer Res Treat 2013; 137: 755-66
Address for correspondence
Publication History
Article published online:
17 June 2021
© 2018. Indian Society of Medical and Paediatric Oncology. This is an open access article published by Thieme under the terms of the Creative Commons Attribution-NonDerivative-NonCommercial-License, permitting copying and reproduction so long as the original work is given appropriate credit. Contents may not be used for commercial purposes, or adapted, remixed, transformed or built upon. (https://creativecommons.org/licenses/by-nc-nd/4.0/).
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2,
Noida-201301 UP, India

| Figure 1:Hair and skin color changes on tablet Pazopanib

| Figure.2:Normal skin color after stopping tablet Pazopoanib

| Figure.3:Normal hair color after stopping tablet Pazopanib
References
- Hurwitz HI, Dowlati A, Saini S, Savage S, Suttle AB, Gibson DM. et al. Phase I trial of pazopanib in patients with advanced cancer. Clin Cancer Res 2009; 15: 4220-7
- Hutson TE, Davis ID, Machiels JP, De Souza PL, Rottey S, Hong BF. et al. Efficacy and safety of pazopanib in patients with metastatic renal cell carcinoma. J Clin Oncol 2010; 28: 475-80
- Sternberg CN, Davis ID, Mardiak J, Szczylik C, Lee E, Wagstaff J. et al. Pazopanib in locally advanced or metastatic renal cell carcinoma: Results of a randomized phase III trial. J Clin Oncol 2010; 28: 1061-8
- Sideras K, Menefee ME, Burton JK, Erlichman C, Bible KC, Ivy SP. Profound hair and skin hypopigmentation in an African American woman treated with the multi-targeted tyrosine kinase inhibitor pazopanib. J Clin Oncol 2010; 28: e312-3
- Bible KC, Suman VJ, Molina JR, Smallridge RC, Maples WJ, Menefee ME. et al. Efficacy of pazopanib in progressive, radioiodine-refractory, metastatic differentiated thyroid cancers: Results of a phase 2 consortium study. Lancet Oncol 2010; 11: 962-72
- de Jonge MJ, Hamberg P, Verweij J, Savage S, Suttle AB, Hodge J. et al. Phase I and pharmacokinetic study of pazopanib and lapatinib combination therapy in patients with advanced solid tumors. Invest New Drugs 2013; 31: 751-9
- Teresa M, González JV, Tlahuel JL, Guerra EC. Hypopigmentation of the Skin and Hair Associated with Targeted Therapies. Journal of Cancerology 2014; 1: 67-72
- Moss KG, Toner GC, Cherrington JM, Mendel DB, Laird AD. Hair depigmentation is a biological readout for pharmacological inhibition of KIT in mice and humans. J Pharmacol Exp Ther 2003; 307: 476-80
- Elhalawani H, Heiba M, Abdel-Rahman O. Risk of distinctive hair changes associated with pazopanib in patients with renal cell carcinoma (RCC) versus patients without RCC: A comparative systematic review and meta-analysis. Clin Genitourin Cancer 2017; 15: e325-35
- Sternberg CN, Hawkins RE, Wagstaff J, Salman P, Mardiak J, Barrios CH. et al. A randomised, double-blind phase III study of pazopanib in patients with advanced and/or metastatic renal cell carcinoma: Final overall survival results and safety update. Eur J Cancer 2013; 49: 1287-96
- GlaxoSmithKline. An open-label, multicentre, randomised phase II study of pazopanib in combination with pemetrexed in first-line treatment of subjects with predominantly non-squamous cell stage IIIB wet/IV non-small cell lung cancer. Bethesda, MD: National Library of Medicine (US); 2000. Available from: https://www.clinicaltrials.gov/ct2/show/NCT 00871403?term¼NCT00871403 and rank¼1.NLM Identifier:NCT00871403. [Last accessed on 2015 Jul 29].
- Johnston SR, Gómez H, Stemmer SM, Richie M, Durante M, Pandite L. et al. A randomized and open-label trial evaluating the addition of pazopanib to lapatinib as first-line therapy in patients with HER2-positive advanced breast cancer. Breast Cancer Res Treat 2013; 137: 755-66


 PDF
PDF  Views
Views  Share
Share

