Role of Insulin-like Growth Factor, Insulin-like Growth Factor Receptors, and Insulin-like Growth Factor-binding Proteins in Ovarian Cancer
CC BY-NC-ND 4.0 · Indian J Med Paediatr Oncol 2017; 38(02): 198-206
DOI: DOI: 10.4103/ijmpo.ijmpo_3_17
Abstract
Insulin and IGFs play an important role in cancer initiation and progression, including ovarian cancer (OC). Epithelial ovarian cancer (EOC) is the most frequent type of OC in women and it is the most lethal gynecological malignancy worldwide. Generally, insulin is associated with metabolism, whereas Insulin like growth factors (IGFs) are involved in cell proliferation. Hence, Insulin-like growth factor binding proteins (IGFBPs) determines the bioavailability of IGFs in circulation. The interplay between these molecules such as insulin, IGFs, IGFBPs and insulin-like growth factor receptor 1 (IGF1R) may be crucial for ovarian cancer cell biology and cancer progression. However, the IGF1R inhibitors exhibiting potent activity on IGF/IGF1R also demonstrated activity against OC cells. The combination therapy of drugs may prove to be beneficial in clinical management of OC. This review describes both molecular and clinical associations between insulin and IGF1 signaling pathways in ovarian cancer. The data was collected using PubMed search engine with the following key words such as ovarian cancer, IGFs, IGFBP, IGF1Rs and ovarian cancer.
Keywords
Insulin-like growth factor - insulin-like growth factor-binding proteins - insulin-like growth factor receptor - obesity - ovarian cancer - prognostic valuePublication History
Article published online:
06 July 2021
© 2017. Indian Society of Medical and Paediatric Oncology. This is an open access article published by Thieme under the terms of the Creative Commons Attribution-NonDerivative-NonCommercial-License, permitting copying and reproduction so long as the original work is given appropriate credit. Contents may not be used for commercial purposes, or adapted, remixed, transformed or built upon. (https://creativecommons.org/licenses/by-nc-nd/4.0/.)
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India
Abstract
Insulin and IGFs play an important role in cancer initiation and progression, including ovarian cancer (OC). Epithelial ovarian cancer (EOC) is the most frequent type of OC in women and it is the most lethal gynecological malignancy worldwide. Generally, insulin is associated with metabolism, whereas Insulin like growth factors (IGFs) are involved in cell proliferation. Hence, Insulin-like growth factor binding proteins (IGFBPs) determines the bioavailability of IGFs in circulation. The interplay between these molecules such as insulin, IGFs, IGFBPs and insulin-like growth factor receptor 1 (IGF1R) may be crucial for ovarian cancer cell biology and cancer progression. However, the IGF1R inhibitors exhibiting potent activity on IGF/IGF1R also demonstrated activity against OC cells. The combination therapy of drugs may prove to be beneficial in clinical management of OC. This review describes both molecular and clinical associations between insulin and IGF1 signaling pathways in ovarian cancer. The data was collected using PubMed search engine with the following key words such as ovarian cancer, IGFs, IGFBP, IGF1Rs and ovarian cancer.
Introduction
Ovarian cancer (OC) is the seventh most common cancer in women.[1] According to the Madras Metropolitan Tumour Registry, OC is the third leading cause of cancer death in women with an age-standardized rate of 7.8/100,000 populations.[2] High mortality rate of OC is due to lack of early symptoms and effective screening procedure. The 5-year disease-free survival for patients with stage IIIc/IV is approximately 27%.[3] The treatment methods available to treat OC include aggressive cytoreductive surgery and combinations of chemotherapy. These led to improved survival rate in treatment group. However, majority of patients who present in stage III/IV at diagnosis will have a high rate of disease relapse and mortality. Patients who are diagnosed at stage I/II have 5-year survival rates of 90/70%.[4]
Insulin-like growth factor (IGF) system plays an extensive role in normal cell as well as in tumor cell biology. IGF system is comprised two peptide ligands (IGF1 and IGF2) which are structurally similar to insulin, a family of six different IGF-binding proteins (IGFBPs, 1–6), and two (IGF1 receptor [IGF1R] and IGF2R) membrane-spanning tyrosine kinase receptors. IGFBPs play a complex role in physiological system which is mainly responsible for protecting IGF in circulation and delivering them to their target site.[5] According to the recent reports, IGFBPs exhibit IGF-dependent and IGF-independent functions as well.[6] In female reproductive system, IGFs play a vital role in follicular development.[7] They are responsible for repairing epithelial tissue after ovulation. During the menstrual cycle, ovarian and granulosa cells undergo rapid proliferation and differentiation which act as a target for insulin and IGFs. Ovarian tumor cells secrete these autocrine/paracrine factors and other cytokines to activate IGF-signaling pathway in epithelial origin which could mediate uncontrolled wound healing mechanism in the ovarian surface epithelium.[8]
IGF family proteins were found to be usually dysregulated in several cancers including prostate, colorectal, breast, and esophageal cancer.[9,10] In addition, exogenous stimulation of OC cell line with IGF1 and IGF2 drives cell proliferation and plays a significant role in the OC pathophysiology and tumorigenesis.[11] In our review, we have discussed the role of IGFs, IGFBPs, and its receptors in OC.
Insulin-Like Growth Factor Receptors and Signaling Pathways
IGF1, IGF2, and insulin bind to IGF1R and insulin receptors (IRs)-A and IR-B. All these receptors belong to the family of tyrosine kinase receptors. Insulin binds to the IRs with high and low affinity to the IGF1R, whereas IGF1 has high affinity to the IGF1R. IGF1R, IR-A, and IR-B form hybrid receptors when they are coexpressed in cells. IGF1 and IGF2 bind to these hybrid receptors with high affinity, whereas insulin binds with less affinity. In contrast to IGF, which is mainly involved in cell growth and survival, insulin is mainly involved in cell metabolic process.[12] IGF2R lacks intracellular tyrosine kinase domain and binds only with IGF2 to regulate the extracellular concentration of IGF2. A study has showed that the unglycosylated IGF2 isoform has increased cell number 1.4-fold over vehicle control and excess of IGF2 isoform had no effect in cell proliferation when compared to mature IGF2.[13] All IGFBPs, including IGFBP2, IGFBP3, and IGFBP5, bind IGF2 isoform similar to mature IGF2.
The IGF-signaling cascade is activated by the ligands. On ligand binding to its receptor IGF1R, the intracellular tyrosine kinase domains are activated by autophosphorylation which subsequently activates the insulin response elements (IR substrate [IRS] 1 and IRS2) through phosphorylation. This will activate similar signaling molecules called adaptor proteins (SHC). This complex activates phosphatidylinositol 3-kinase (PI3K) which converts phosphatidylinositol 3, 4 phosphate into phosphatidylinositol 3, 4, 5 phosphate. This secondary messenger activates Akt phosphorylation. Tuberous sclerosis protein 1/2 is downstream of Akt which inhibits mammalian target of rapamycin complex and regulate cell proliferation.[14] This network inhibits apoptosis through phosphorylation of BCL2 Associated Agonist Of Cell Death (BAD) and FKHR. This signaling pathway can also activate SHC and GRB2 adaptor proteins which activate mitogen-activated protein kinase (MAPK) pathway resulting in cell proliferation.[15] Phosphatase And Tensin Homolog (PTEN) inhibits both PI3K and Akt. IGFBPs modulate the bioavailability of IGF1 and IGF2 to their receptors. IGFBPs also exert several IGF-independent effects through direct interaction with cell membrane-bound proteins, such as integrins. In IGF-independent function, IGFBPs can inhibit nuclear factor-κB (NFκB) and promote cell death through caspase-8 activation.[16]
IGF1R-signaling pathway promotes cell proliferation through activation of MAPK pathway and blockade of apoptosis pathway by inhibition of a proapoptotic protein BAD.[17] It also inhibits apoptosis by phosphorylating apoptosis signal-regulating kinase 1 (ASK1) which is a mitogen-activated protein kinase kinase kinase. ASK1 is required for tumor necrosis factor-α-induced apoptosis and also oxidative stress-induced programmed cell death. Inhibition of ASK1 by IGF1R can activate cell proliferation, survival, and inhibit cell death.[18] The recent studies have reported that a cross talk happens between a prosurvival protein such as aurora kinase A and IGF1/PI3K/Akt pathway at Akt activation.[19] The representation of IGF1-signaling network has given in Figure 1.
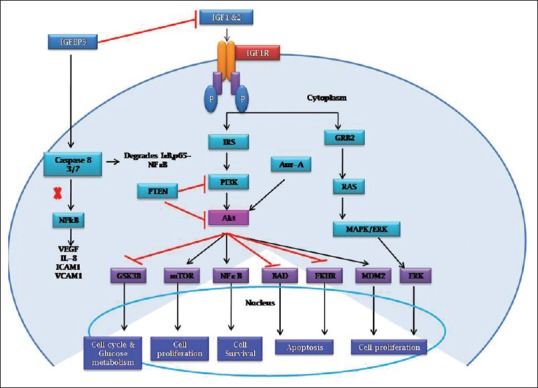
| Figure 1:The insulin-like growth factor 1-signaling pathway in cells. Insulin-like growth factor 1 activates both phosphatidylinositol 3-kinase/Akt and Ras/mitogen-activated protein kinase pathway resulting in cell proliferation, increased protein synthesis, and increased glucose metabolism. Phosphatidylinositol 3-kinase/Akt activates nuclear factor-κB for cell survival and inhibits apoptosis through inhibition of BAD and FKHR. Insulin-like growth factor-binding proteins modulate the bioavailability of insulin-like growth factor through direct binding in the extracellular space. Insulin-like growth factor-binding proteins also exert several insulin-like growth factor-independent effects through direct interaction with cell membrane-bound proteins, such as integrins
Clinical Importance of Insulin-Like Growth Factors in Ovarian Cancer Risk and Progression
All IGF-signaling system components are expressed in OC. These components have been shown to stimulate cell proliferation, invasive, and angiogenic activity of OC cells.[20] In contrast, these effects were suppressed by IGFBPs.[21] Several studies have revealed the prognostic value of IGF system components’ expression in OC.[22]
Obesity, insulin-like growth factors, and risk of ovarian cancer
Obesity is associated with poor survival in many cancers including breast, colorectal, and prostate cancer. Obese women may have aggressive tumors because excessive deposition of adipose tissue can upregulate cell proliferation and metastasis pathways.[23] It has been found that obesity is associated with hyperinsulinemia which results in increased growth hormone receptor expression which in turn increases hepatic production of IGF1, resulting in increased free IGF1 levels. High IGF1 ultimately activates downstream signaling pathway including cell proliferation, invasion, and metastasis.[24] White adipose tissue produces a protein called leptin which is encoded by a gene ob. This protein acts as a growth factor in a number of cancer cell lines including breast, endometrial, and prostate cancers. It has been shown to activate MAPK and NFκB pathway causing apoptosis resistance in colon, breast, and prostate cancer.[25]
Obesity also increases the risk of ovarian, endometrial, and estrogen receptor/progesterone receptor (ER/PR)-positive postmenopausal breast cancer.[26] A tissue microarray study reported Ob-R overexpression in 59.2% epithelial OC (EOC) and was associated with poor prognosis. A case-cohort study demonstrated a positive correlation between serum insulin levels and endometrioid adenocarcinoma which was independent of serum estradiol concentration. This correlation was stronger in obese women than in nonobese women. In contrast, there is an inverse relationship between free serum IGF1 and endometrioid adenocarcinoma.[27] ER-positive breast cancer risk was found to be associated with circulating IGF.[28]
Insulin-like growth factor-binding proteins in ovarian cancer
Six different IGFBPs (IGFBP1, IGFBP2, IGFBP3, IGFBP4, IGFBP5, and IGFBP6) have been identified by molecular cloning of their complementary DNAs from rat and human tissues.[29] In four OC cell lines (EFO-21, EFO-27, MFO-35, and MFO-36), IGFBP3, IGFBP4, and IGFBP6 were often expressed while IGFBP2 followed by IGFBP5 was less commonly expressed; these cells did not express IGFBP1.[30] Using quantitative serum proteomic study, it was reported that serum from patients with OC had differential expression of IGFBP3 and IGFBP6.[31]
Insulin-like growth factor-binding protein 1
IGFBP1 was the first member of IGFBP family which has major effects on implantation and trophoblast invasion.[32] It is the major binding protein in the secretory endometrium, decidua of placenta, and Arias-Stella glands of miscarriage material. Overexpression of IGFBP1 protein and messenger RNA (mRNA) was seen in ovarian clear cell adenocarcinoma (90.3%) compared with nonclear cell adenocarcinomas such as serous adenocarcinoma, endometrioid adenocarcinoma, and mucinous adenocarcinoma. However, there was no association between the tumor node metastasis stage and IGFBP1 expression.[33]
Insulin-like growth factor-binding protein 2
IGFBP2 was the less abundant circulating IGFBPs. In an in vitro study, SKOV3 OC cell line has showed very low-level expression of endogenous IGFBP2. Hence, IGFBP2 overexpressing SKVO3 cells were derived by transfection of IGFBP2 overexpressing clones. The invasiveness of the IGFBP2 overexpressing cells was higher than the vector control. This proposes that recruitment of IGFBP2 is an important step in the penetration of the extracellular matrix (ECM) by OC cells.[34] Lee et al. and Flyvbjerg et al. proposed that IGFBP2 was overexpressed in invasive ovarian carcinomas compared to borderline OCs and normal ovarian tissues in western blot and tissue microarray analysis.[34,35] Moreover, increased serum IGFBP2 level indicates a poor prognosis.[36] A high level of IGFBP2 is present in ovarian tumor cystic fluid. These findings represent that high amount of IGFBP2 production by the tumor microenvironment leads to increased levels in cystic fluid to further cause spreading of malignancy. These studies demonstrate that autocrine-signaling network exists in OC microenvironment for IGF1. This will aid tumor cell proliferation in the ovary and further progression of the disease.[37,38]
Insulin-like growth factor-binding protein 3
IGFBP3 binds to both IGF1 and IGF2 ligands and forms an acid-labile subunit. This is the major circulating forms of IGFBPs in human serum and represents the prime regulator of IGF half-life in the circulation. It has both IGF-dependent and IGF-independent functions in human biological system. IGFBP3 inhibits cell proliferation and migration and induces cell death. It is transcriptionally regulated by p53[39] and will act as a significant regulator of cell survival through IGF-independent mechanisms. The mutant IGFBP3 (lack of IGF1-binding site) when transfected into insulin-secreting RIN-m5F cells has been shown to induce apoptosis and inhibit cell growth in dose- and time-dependent manner than control cells.[40]
Insulin-like growth factor-binding protein 4
The gene IGFBP4 is mapped to chromosome region 17q12-q21.1 by in situ hybridization.[41] As with other IGFBPs, it also has ligand-independent activity. It is primarily secreted by the liver and present in all body fluids. It is also expressed by a number of organs including ovaries. In the ovary, in response to estrogen, it is upregulated and involved in the follicle selection.[42] The diet which contains flaxseed reduced the mRNA expression of IGFBP4 in preneoplastic hen ovaries due to its antiestrogenic effect.[43] The EOC transcriptome was analyzed using both early- and late-stage sample set by RNA-Seq and identified that IGFBP4 is highly expressed across all stages of EOC.[44] Zhu et al. had shown IGFBP4 to inhibit Wnt signaling through interaction with Frizzled-8 and low-density lipoprotein receptor-related protein 6 (which is a Wnt co-receptor) in cardiomyocytes.[45] IGFBP4 was also found to be differentially expressed in OCs, and additionally, serum IGFBP4 levels were elevated in OC patients even earlier than cancer antigen 125.[44]
Insulin-like growth factor-binding protein 5
In both in vitro and in vivo systems, IGFBP5 functions as an antiangiogenic protein by inhibiting endothelial cell proliferation and migration. It also reduced the expression of phosphorylated Akt and phosphorylated endothelial nitric oxide synthase (eNOS) in human umbilical vascular endothelial cells which might be an IGF1-independent action. Both Akt and eNOS play an important role in angiogenesis when activated by vascular endothelial growth factor.[21] IGFBP5 expression was significantly higher in high-grade serous adenocarcinoma compared to low-grade serous carcinoma, serous borderline tumors, benign cysts, and normal ovarian epithelial surface using immunohistochemical and tissue microarray analysis. Its expression was low to absent in ovarian clear cell carcinoma and mucinous carcinomas, suggesting that IGFBP5 may play a role in the genesis of high-grade serous tumor but not in the mucinous or clear cell tumor.[36]
Insulin-like growth factor-binding protein 6
IGFBP6 differs from other family member proteins because it binds preferentially with IGF2 over IGF1. It has both IGF2-dependent and IGF2-independent functions. In the cell migration assay, IGFBP6 increases the migration of SKOV3 OC cells in the absence of IGF2, whereas in HEY, OC cells showed only basal level of migration without IGF2. Addition of IGF2 to increases migration of the HEY cells. This report suggests that in HEY cells, migration is IGF dependent. IGFBP6-dependent changes in migration of both cell lines were accompanied by Ras/MAPK-signaling pathway activation. Thus, this cannot explain the opposite direction of the migratory responses. IGFBP6 inhibits the actions of IGF2 and angiogenesis by an IGF-independent pathway.[46] These may contribute to its antitumorigenic effects.[47] A microarray study also reported that IGFBP6 mRNA levels were lower in OC tissue compared with normal ovarian tissue.[48] This may reflect derepression of IGF2 action by decreased IGFBP6, but levels were not confirmed by an independent assay. Plasma levels of IGFBP6 in OC have been found to be downregulated in patients with OC compared to those without the cancer.[49]
Pregnancy-associated plasma protein A and insulin-like growth factor system in ovarian cancer
In 1974, Lin et al. identified human pregnancy-associated plasma protein A (PAPPA) in the plasma of pregnant women.[50] Conover et al. was subsequently shown a novel proteinase activity of PAPPA responsible for cleavage of IGFBP4 in ovarian follicular fluid.[51] A strong PAPPA and IGF connection was found in ovarian follicular growth and selection in multiple species. The proteolytic degradation of IGFBP4 is a common feature of preovulatory follicles from human, bovine, rodent, equine, porcine, and possum follicles. This is due to decreased IGFBP gene expression as well as to increased PAPPA activity.[51,52,53,54] This proteolytic degradation is IGF dependent and determines the IGF bioavailability which is an important determinant of follicular fate.[53,54,55,56] Thus, PAPPA increases bioavailability and mitogenic effect of IGF by an autocrine/paracrine regulation. Binding of PAPPA to the proteoglycans through PAPPA modules such as SCR3 and SCR4 on the cell surface causes proteolytic cleavage of IGFBP4 to occur in proximity of the IGF receptor. This will increase the probability that released IGF leads to receptor signaling.[57,58] The other substrates for PAPPA are IGFBP2[59] and IGFBP5.[60] This cleavage will occur in an IGF-independent manner.[60] Hence, these IGFBPs are also cleaved by other proteinases such as PAPPA2, a structural homolog of PAPPA. However, physiological cleavage of IGFBP4 can be limited to PAPPA.[58,61] Compared with PAPPA, PAPPA2 has currently been much less studied.[62]
PAPPA increases bioavailability of IGF and promotes ovarian tumor growth through degradation of IGFBP4. In an in vitro study using human, SKOV3 ovarian carcinoma cell line reported that clones with increased PAPPA expression showed promoted anchorage-independent growth compared with clones overexpressing mutant PAPPA and vector controls in soft agar assays. SKOV3 clones with the highest PAPPA expression and IGFBP proteolytic activity showed increased cell invasion in Matrigel assay. In an in vivo study, PAPPA overexpressing SKOV3 clones significantly accelerated tumor growth rates compared with mutant PAPPA and controls. This also favors angiogenesis and neovascularization months before obvious tumor development.[63] The ascitic fluid of OC patients showed 46-fold higher PAPPA levels as compared to serum (P < 0 href="https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5582559/#ref64" rid="ref64" class=" bibr popnode tag_hotlink tag_tooltip" id="__tag_635950120" role="button" aria-expanded="false" aria-haspopup="true" xss=removed>64] This was supported by the finding that ascites contained more cleaved form of IGFBP4 than intact.[64,65] In addition, the expression of irreversible PAPPA inhibitor such as pro-major basic protein has showed to be increased in conditioned medium from short-term ovarian tumor cultures and transformed ovarian epithelial cells.[66] The mRNA expression of PAPPA correlated with poor patient outcome in ovarian tumors.[65]
Importance of Insulin-Like Growth Factors in Ovarian Cancer
IGF1R, a tyrosine kinase receptor, plays an important role in cancer biology. This has been well studied in cell culture and in animal models and found to play a role in tumor transformation, progression, and metastasis.
Role of insulin-like growth factors in ovarian cancer growth and progression
Receptor tyrosine kinase (RTK) was proven to play an important role in the formation and progression of cancer in human. Among RTK, IGF1R has been well studied in cell culture models as well as in animal models and confirmed its role in tumor transformation, disease progression, protection from cell death, and spread to distance organs. A relative balance between IGF-signaling network proteins plays a vital role in maintaining healthy ovarian tissue. This pathway was dysregulated in many cancers including OC. A study reported that serum-free high IGF1 levels increase the risk of developing OC and tumor progression in women <55 href="https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5582559/#ref67" rid="ref67" class=" bibr popnode tag_hotlink tag_tooltip" id="__tag_635950124" role="button" aria-expanded="false" aria-haspopup="true" xss=removed>67,68] Murine ovary studies reported altered ovarian surface epithelium leading to hyperplasia in the ovaries and altered ECM deposition when cultured in the presence of insulin or IGF1.[69]
Estrogen plays an important role in OC growth and progression in ERα-positive cancers. Estrogen reacts with target genes through AP1 site which exist in promoter of c-Myc and IGF1 genes.[70] They have studied estrogen (estradiol, E2) and raloxifene (selective estrogen modulator) transcriptional response in CaOV3 (ERα positive), OVCAR-3 (ERα positive), and A2780 (ERα negative) OC cells. These results demonstrate that SRC-1 (IGF1 signaling pathway adapter protein) is a necessary determinant for the E2-stimulated cell cycle progression in OC cells. This report showed that E2 enhanced the c-Myc expression in ERα-positive cells but not in ERα-negative cells through transcription control. In contrast to E2, raloxifene did not have much effect in the transcription of c-Myc. Estrogen also modulates the expression of IGFBP3, IGFBP4, and IGFBP5 in ERα-positive cell culture but not IGFBP1, IGFBP2, and IGFBP6. This study indicates that IGFBPs can be a good predictive marker in ER-positive cases and help identify patients who are likely to respond to the treatment.[71]
Aberrant insulin-like growth factor 1 receptor signaling in ovarian cancer
DOV-13°C cells are highly sensitive to anoikis which expresses high levels of protein tyrosine kinase 6 (PTK6). This has not been detected in normal ovarian surface epithelial cells. Interestingly, PTK6 mediates its role through activating downstream signaling molecules by autophosphorylation of IGF1R at tyrosine 1131, 1135, and 1136 in the presence of IGF1 which is responsible for anchorage-independent cell survival in OC.[72] Another important anchorage-independent growth in OC is mediated by STAT3. Receptor of activated C kinase 1 forms a complex with STAT3 in cytosol which will be recruited to the IR-IGF1R heterodimers. This complex also recruits Janus Kinase's specifically for IR-IGF1R-mediated phosphorylation and subsequent to the receptor activation.[73]
PRKCZ gene encodes a protein called protein kinase C (PKC) zeta (PKCζ) which belongs to the family of an enzyme serine-threonine kinase. The PAR polarity complex comprises PAR3, PAR6, and atypical PKC (PKCζ and PKCι). These polarity complexes regulate epithelial polarization through their interactions with the cytoskeleton and adhesion proteins. These usually exhibit alterations in cancer that drive tumorigenesis, but they are predominantly associated with tumor progression. However, cross talk between polarity complexes and other signaling pathways seems to drive tumorigenesis.[74] In addition, the free IGF1 level will activate PKCζ in vascular smooth muscle cells. IGF1 also phosphorylates SHPS-1 kinase receptor which is a primary target of IGF1 in hyperglycemic conditions. Activated SHPS-1 forms a multiprotein-signaling complex with SHP-2/integrin-linked kinase (ILK)/PKCζ/vimentin. ILK in response to IGF1 recruits PKCζ to SHPS-1/vimentin complex. PKCζ phosphorylates vimentin at serine residues which opens a binding site for Receptor protein tyrosine phosphatase beta (RPTPβ). In conjunction with IGF1R, IGFBP2 binds with RPTPβ and stimulates the association of serine-phosphorylated vimentin/RPTPβ, resulting in RPTPβ polymerization. This leads to activation of the downstream signaling cascade such as inactivation of PTEN by increased PTEN tyrosine phosphorylation, activation of Akt, and cell proliferation by its phosphatase activity.[75,76] This PKCζ is known to plays a role in OC cell viability, proliferation, and migration. Seto and Andrulis reported that overexpression of PKCζ stimulated cell proliferation in SKOV3 OC cells by increasing either translation or stability of IGF1R and possibly leading to constitutive activation of IGF1-signaling cascade that results in transcriptional repression of ITGB3 and increases cell survival.[77] A microarray analysis of serous OC showed that IGF2 was overexpressed in most of the cases analyzed compared to normal tissue.[78] Loss of imprinting (LOI) is not a frequent event in serous OCs, and there is no association between elevated IGF2 expression and LOI in OC.[79] The study reported that at an early stage of ovarian cancer showed hypomethylation of IGF2 differential methylation region and/or site 1 CCCTC-binding factor (CTCF) hypermethylation, whereas site 6 of CTCF was hypermethylated in advanced stage of disease. These data suggest that elevation of IGF2 levels occurs in the absence of IGF2 LOI. This could be used as a diagnostic method to detect the disease at early stage.[80]
Prognostic Value
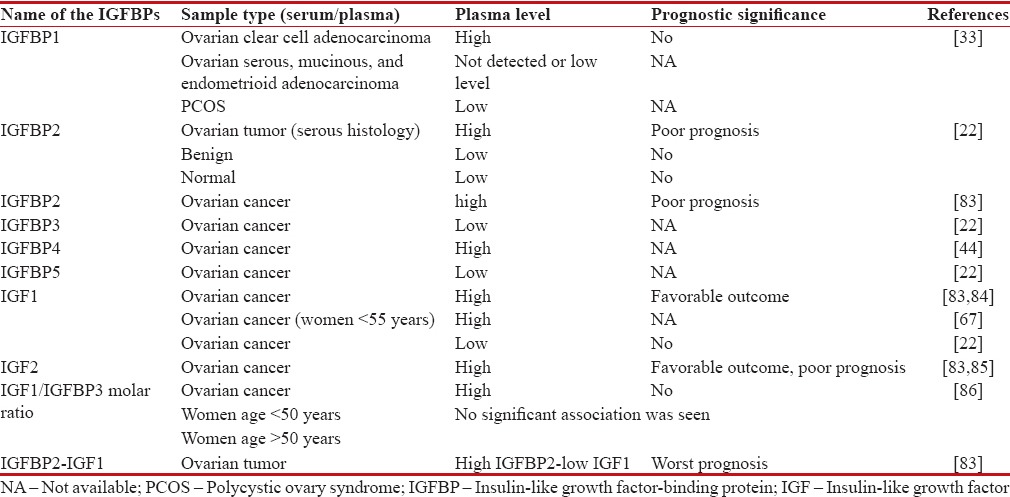
A study reported that high expression of IGF1R was predominantly observed in EOC than in benign tumors as well as in normal ovary.[81] A methylation study has reported that malignant ovarian tumor is three times more methylated on IGFBP3 promoter region than low malignant tumor after adjusting for age.[82] The prognostic value of IGF proteins are shown in Table 1.
Table 1
Prognostic value of insulin-like growth factor-signaling proteins
|
Inhibition of Insulin-Like Growth Factor Network
IGF system is activated in many cancers including OC, which suggests that IGF network system is a promising therapeutic target in OC. An epidemiological study showed that high animal protein in the diet induces IGF1, insulin, and aging and is a major cause of mortality for people at age of 45–65 years. Restriction of IGF1 level by reducing the calorie intake and taking more of plant proteins than animal proteins relatively reduces cancer risk.[87] The drugs that are currently in clinical trial and used for the treatment of cancer were given in Table 2.
Table 2
List of insulin-like growth factor-signaling pathway inhibitory drugs

|
Numerous compounds were screened for IGF1R inhibitory studies. However, IGF1R's structural similarity with IRs makes it difficult in the development of IGF1R inhibitors exclusively blocking IGF1 signaling. Drug compounds targeting IGF1R also inhibit insulin pathway which contributes to hyperglycemia in clinical trials.[88] This indicates that we need new potential targets in IGF network to treat cancer.
The following reasons are proposed for the failure of IGF1R-targeted therapy in cancer:[89]
- Cells are rendered resistant to IGF1R therapy due to mutations in PI3K which will constitutively activate Akt and is downstream to IGF1R
- Targeted therapy inhibits differentiation of hematopoietic precursors and neuronal cells
- Replacement of IGF1R signaling by IR-A
- Development of adaptive resistance
- Nuclear localization of IGF1R which hinders the accessibility of IGF1R-directed antibodies
- Heterogeneous nature of the tumor
- Cross talk between regulatory pathways and potential to evade regulatory checkpoints.
Alternate targets in the IGFR pathways include PAPPA and aurora kinase A. PAPPA is a protease that cleaves IGFBPs and increases free IGF level. This suggests that PAPPA may serve as a potential target in the IGF-signaling pathway.[90] The antitumor efficacy of monoclonal PAPPA antibody (mAb-PA) was examined in multiple primary patient ovarian tumor graft models, and the tumor response was depending on PAPPA expression. Hence, the addition of mAb-PA to standard platinum chemotherapy sensitized platinum-resistant tumor. This also inhibited the development and progression and induced the regression of ovarian tumor.[65] Aurora kinase A regulates mitosis in cell division which has been dysregulated in many tumors. This kinase interacts with IGF1/PI3K/Akt pathway and activates Akt. A study report suggests that inhibition of both aurora kinase A and PI3K gives a synergistic effect in cancer treatment.[19] Mitsuhashi et al. reported on the effects of 4–6 weeks of preoperative administration of metformin to endometrial cancer patients, showing significant reduction in insulin, glucose, IGF1, leptin levels, and thymidine uptake activity of serum.[91] This suggests the potential of metformin in endometrial cancers.
Conclusion
Recent progress in research contributes better understanding of OC biology for identification and optimization of reliable biomarkers. The IGF family proteins are thought to play a vital role in cancer development and progression, but the exact molecular mechanisms have not yet been revealed. However, some members of the IGF family protein expression have shown promise as prognostic markers in OC but need more validation in larger number of samples. To date, IGFs can neither be used as biomarkers for OC screening nor as predictive marker for decision on OC patient therapy. The current IGF1R inhibitors have showed only limited benefit in OCs.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Ferlay J, Steliarova-Foucher E, Lortet-Tieulent J, Rosso S, Coebergh JW, Comber H, et al. Cancer incidence and mortality patterns in Europe: Estimates for 40 countries in 2012. Eur J Cancer 2013;49:1374-403.
- Swaminathan R, Shanta V. Population based Cancer Registry, Chennai Cancer Institute (WIA), Adyar, Chennai. Individual Registry Write-up: 2006-2008; 2010. p. 165-83.
- Kaku T, Ogawa S, Kawano Y, Ohishi Y, Kobayashi H, Hirakawa T, et al. Histological classification of ovarian cancer. Med Electron Microsc 2003;36:9-17.
- Jelovac D, Armstrong DK. Recent progress in the diagnosis and treatment of ovarian cancer. CA Cancer J Clin 2011;61:183-203.
- Weroha SJ, Haluska P. The insulin-like growth factor system in cancer. Endocrinol Metab Clin North Am 2012;41:335-50, vi.
- Forbes BE, McCarthy P, Norton RS. Insulin-like growth factor binding proteins: A structural perspective. Front Endocrinol (Lausanne) 2012;3:38.
- Oosterhuis GJ, Vermes I, Lambalk CB, Michgelsen HW, Schoemaker J. Insulin-like growth factor (IGF)-I and IGF binding protein-3 concentrations in fluid from human stimulated follicles. Hum Reprod 1998;13:285-9.
- Brokaw J, Katsaros D, Wiley A, Lu L, Su D, Sochirca O, et al. IGF-I in epithelial ovarian cancer and its role in disease progression. Growth Factors 2007;25:346-54.
- Douglas JB, Silverman DT, Pollak MN, Tao Y, Soliman AS, Stolzenberg-Solomon RZ. Serum IGF-I, IGF-II, IGFBP-3, and IGF-I/IGFBP-3 molar ratio and risk of pancreatic cancer in the prostate, lung, colorectal, and ovarian cancer screening trial. Cancer Epidemiol Biomarkers Prev 2010;19:2298-306.
- Burger AM, Leyland-Jones B, Banerjee K, Spyropoulos DD, Seth AK. Essential roles of IGFBP-3 and IGFBP-rP1 in breast cancer. Eur J Cancer 2005;41:1515-27.
- Conover CA, Hartmann LC, Bradley S, Stalboerger P, Klee GG, Kalli KR, et al. Biological characterization of human epithelial ovarian carcinoma cells in primary culture: The insulin-like growth factor system. Exp Cell Res 1998;238:439-49.
- Cheng AJ, Chen LC, Chien KY, Chen YJ, Chang JT, Wang HM, et al. Oral cancer plasma tumor marker identified with bead-based affinity-fractionated proteomic technology. Clin Chem 2005;51:2236-44.
- Marks AG, Carroll JM, Purnell JQ, Roberts CT Jr. Plasma distribution and signaling activities of IGF-II precursors. Endocrinology 2011;152:922-30.
- Kennedy SG, Wagner AJ, Conzen SD, Jordán J, Bellacosa A, Tsichlis PN, et al. The PI 3-kinase/Akt signaling pathway delivers an anti-apoptotic signal. Genes Dev 1997;11:701-13.
- Pouysségur J, Volmat V, Lenormand P. Fidelity and spatio-temporal control in MAP kinase (ERKs) signalling. Biochem Pharmacol 2002;64:755-63.
- Han J, Jogie-Brahim S, Harada A, Oh Y. Insulin-like growth factor-binding protein-3 suppresses tumor growth via activation of caspase-dependent apoptosis and cross-talk with NF-κB signaling. Cancer Lett 2011;307:200-10.
- Peruzzi F, Prisco M, Dews M, Salomoni P, Grassilli E, Romano G, et al. Multiple signaling pathways of the insulin-like growth factor 1 receptor in protection from apoptosis. Mol Cell Biol 1999;19:7203-15.
- Galvan V, Logvinova A, Sperandio S, Ichijo H, Bredesen DE. Type 1 insulin-like growth factor receptor (IGF-IR) signaling inhibits apoptosis signal-regulating kinase 1 (ASK1). J Biol Chem 2003;278:13325-32.
- Yao JE, Yan M, Guan Z, Pan CB, Xia LP, Li CX, et al. Aurora-A down-regulates IkappaBalpha via Akt activation and interacts with insulin-like growth factor-1 induced phosphatidylinositol 3-kinase pathway for cancer cell survival. Mol Cancer 2009;8:95.
- Shao M, Hollar S, Chambliss D, Schmitt J, Emerson R, Chelladurai B, et al. Targeting the insulin growth factor and the vascular endothelial growth factor pathways in ovarian cancer. Mol Cancer Ther 2012;11:1576-86.
- Rho SB, Dong SM, Kang S, Seo SS, Yoo CW, Lee DO, et al. Insulin-like growth factor-binding protein-5 (IGFBP-5) acts as a tumor suppressor by inhibiting angiogenesis. Carcinogenesis 2008;29:2106-11.
- Baron-Hay S, Boyle F, Ferrier A, Scott C. Elevated serum insulin-like growth factor binding protein-2 as a prognostic marker in patients with ovarian cancer. Clin Cancer Res 2004;10:1796-806.
- Wolin KY, Carson K, Colditz GA. Obesity and cancer. Oncologist 2010;15:556-65.
- Farghaly SA. Ovarian cancer in obese women: Risk and optimal medical and surgical treatment options. Womens Health (Lond) 2015;11:261-3.
- Rose DP, Komninou D, Stephenson GD. Obesity, adipocytokines, and insulin resistance in breast cancer. Obes Rev 2004;5:153-65.
- Benedetto C, Salvagno F, Canuto EM, Gennarelli G. Obesity and female malignancies. Best Pract Res Clin Obstet Gynaecol 2015;29:528-40.
- Gunter MJ, Hoover DR, Yu H, Wassertheil-Smoller S, Manson JE, Li J, et al. Aprospective evaluation of insulin and insulin-like growth factor-I as risk factors for endometrial cancer. Cancer Epidemiol Biomarkers Prev 2008;17:921-9.
- Endogenous Hormones and Breast Cancer Collaborative Group, Key TJ, Appleby PN, Reeves GK, Roddam AW. Insulin-like growth factor 1 (IGF1), IGF binding protein 3 (IGFBP3), and breast cancer risk: Pooled individual data analysis of 17 prospective studies. Lancet Oncol 2010;11:530-42.
- Shimasaki S, Ling N. Identification and molecular characterization of insulin-like growth factor binding proteins (IGFBP-1, -2, -3, -4, -5 and -6). Prog Growth Factor Res 1991;3:243-66.
- Hofmann J, Wegmann B, Hackenberg R, Kunzmann R, Schulz KD, Havemann K. Production of insulin-like growth factor binding proteins by human ovarian carcinoma cells. J Cancer Res Clin Oncol 1994;120:137-42.
- Lin B, White JT, Wu J, Lele S, Old LJ, Hood L, et al. Deep depletion of abundant serum proteins reveals low-abundant proteins as potential biomarkers for human ovarian cancer. Proteomics Clin Appl 2009;3:853-61.
- Fowler DJ, Nicolaides KH, Miell JP. Insulin-like growth factor binding protein-1 (IGFBP-1): A multifunctional role in the human female reproductive tract. Hum Reprod Update 2000;6:495-504.
- Sugita S, Morishita Y, Kano J, Furuya S, Shiba-Ishii A, Noguchi M. IGFBP-1 is expressed specifically in ovarian clear cell adenocarcinoma. Histopathology 2011;58:729-38.
- Lee EJ, Mircean C, Shmulevich I, Wang H, Liu J, Niemistö A, et al. Insulin-like growth factor binding protein 2 promotes ovarian cancer cell invasion. Mol Cancer 2005;4:7.
- Flyvbjerg A, Mogensen O, Mogensen B, Nielsen OS. Elevated serum insulin-like growth factor-binding protein 2 (IGFBP-2) and decreased IGFBP-3 in epithelial ovarian cancer: Correlation with cancer antigen 125 and tumor-associated trypsin inhibitor. J Clin Endocrinol Metab 1997;82:2308-13.
- Wang H, Rosen DG, Wang H, Fuller GN, Zhang W, Liu J. Insulin-like growth factor-binding protein 2 and 5 are differentially regulated in ovarian cancer of different histologic types. Mod Pathol 2006;19:1149-56.
- Karasik A, Menczer J, Pariente C, Kanety H. Insulin-like growth factor-I (IGF-I) and IGF-binding protein-2 are increased in cyst fluids of epithelial ovarian cancer. J Clin Endocrinol Metab 1994;78:271-6.
- Kanety H, Kattan M, Goldberg I, Kopolovic J, Ravia J, Menczer J, et al. Increased insulin-like growth factor binding protein-2 (IGFBP-2) gene expression and protein production lead to high IGFBP-2 content in malignant ovarian cyst fluid. Br J Cancer 1996;73:1069-73.
- Torng PL, Lin CW, Chan MW, Yang HW, Huang SC, Lin CT. Promoter methylation of IGFBP-3 and p53 expression in ovarian endometrioid carcinoma. Mol Cancer 2009;8:120.
- Chen X, Ferry RJ Jr. Novel actions of IGFBP-3 on intracellular signaling pathways of insulin-secreting cells. Growth Horm IGF Res 2006;16:41-8.
- Tonin P, Ehrenborg E, Lenoir G, Feunteun J, Lynch H, Morgan K, et al. The human insulin-like growth factor-binding protein 4 gene maps to chromosome region 17q12-q21.1 and is close to the gene for hereditary breast-ovarian cancer. Genomics 1993;18:414-7.
- Durai R, Davies M, Yang W, Yang SY, Seifalian A, Goldspink G, et al. Biology of insulin-like growth factor binding protein-4 and its role in cancer (review). Int J Oncol 2006;28:1317-25.
- Dikshit A, Gao C, Small C, Hales K, Hales DB. Flaxseed and its components differentially affect estrogen targets in pre-neoplastic hen ovaries. J Steroid Biochem Mol Biol 2016;159:73-85.
- Mosig RA, Lobl M, Senturk E, Shah H, Cohen S, Chudin E, et al. IGFBP-4 tumor and serum levels are increased across all stages of epithelial ovarian cancer. J Ovarian Res 2012;5:3.
- ;Zhu W, Shiojima I, Ito Y, Li Z, Ikeda H, Yoshida M, et al. IGFBP-4 is an inhibitor of canonical Wnt signalling required for cardiogenesis. Nature 2008;454:345-9.
- Zhang C, Lu L, Li Y, Wang X, Zhou J, Liu Y, et al. IGF binding protein-6 expression in vascular endothelial cells is induced by hypoxia and plays a negative role in tumor angiogenesis. Int J Cancer 2012;130:2003-12.
- Yang Z, Bach LA. Differential effects of insulin-like growth factor binding protein-6 (IGFBP-6) on migration of two ovarian cancer cell lines. Front Endocrinol (Lausanne) 2015;5:231.
- Bahrani-Mostafavi Z, Tickle TL, Zhang J, Bennett KE, Vachris JC, Spencer MD, et al. Correlation analysis of HOX, ErbB and IGFBP family gene expression in ovarian cancer. Cancer Invest 2008;26:990-8.
- Gunawardana CG, Kuk C, Smith CR, Batruch I, Soosaipillai A, Diamandis EP. Comprehensive analysis of conditioned media from ovarian cancer cell lines identifies novel candidate markers of epithelial ovarian cancer. J Proteome Res 2009;8:4705-13.
- Lin TM, Halbert SP, Spellacy WN. Measurement of pregnancy-associated plasma proteins during human gestation. J Clin Invest 1974;54:576-82.
- Conover CA, Oxvig C, Overgaard MT, Christiansen M, Giudice LC. Evidence that the insulin-like growth factor binding protein-4 protease in human ovarian follicular fluid is pregnancy associated plasma protein-A. J Clin Endocrinol Metab 1999;84:4742-5.
- Mazerbourg S, Overgaard MT, Oxvig C, Christiansen M, Conover CA, Laurendeau I, et al. Pregnancy-associated plasma protein-A (PAPP-A) in ovine, bovine, porcine, and equine ovarian follicles: Involvement in IGF binding protein-4 proteolytic degradation and mRNA expression during follicular development. Endocrinology 2001;142:5243-53.
- Hourvitz A, Kuwahara A, Hennebold JD, Tavares AB, Negishi H, Lee TH, et al. The regulated expression of the pregnancy-associated plasma protein-A in the rodent ovary: A proposed role in the development of dominant follicles and of corpora lutea. Endocrinology 2002;143:1833-44.
- Juengel JL, Haydon LJ, Mester B, Thomson BP, Beaumont M, Eckery DC. The role of IGFs in the regulation of ovarian follicular growth in the brushtail possum (Trichosurus vulpecula). Reproduction 2010;140:295-303.
- Mazerbourg S, Bondy CA, Zhou J, Monget P. The insulin-like growth factor system: A key determinant role in the growth and selection of ovarian follicles? a comparative species study. Reprod Domest Anim 2003;38:247-58.
- Spicer LJ. Proteolytic degradation of insulin-like growth factor binding proteins by ovarian follicles: A control mechanism for selection of dominant follicles. Biol Reprod 2004;70:1223-30.
- Laursen LS, Overgaard MT, Weyer K, Boldt HB, Ebbesen P, Christiansen M, et al. Cell surface targeting of pregnancy-associated plasma protein A proteolytic activity. Reversible adhesion is mediated by two neighboring short consensus repeats. J Biol Chem 2002;277:47225-34.
- Laursen LS, Kjaer-Sorensen K, Andersen MH, Oxvig C. Regulation of insulin-like growth factor (IGF) bioactivity by sequential proteolytic cleavage of IGF binding protein-4 and -5. Mol Endocrinol 2007;21:1246-57.
- Monget P, Mazerbourg S, Delpuech T, Maurel MC, Manière S, Zapf J, et al. Pregnancy-associated plasma protein-A is involved in insulin-like growth factor binding protein-2 (IGFBP-2) proteolytic degradation in bovine and porcine preovulatory follicles: Identification of cleavage site and characterization of IGFBP-2 degradation. Biol Reprod 2003;68:77-86.
- Laursen LS, Overgaard MT, Søe R, Boldt HB, Sottrup-Jensen L, Giudice LC, et al. Pregnancy-associated plasma protein-A (PAPP-A) cleaves insulin-like growth factor binding protein (IGFBP)-5 independent of IGF: Implications for the mechanism of IGFBP-4 proteolysis by PAPP-A. FEBS Lett 2001;504:36-40.
- Ning Y, Schuller AG, Conover CA, Pintar JE. Insulin-like growth factor (IGF) binding protein-4 is both a positive and negative regulator of IGF activity in vivo. Mol Endocrinol 2008;22:1213-25.
- Overgaard MT, Boldt HB, Laursen LS, Sottrup-Jensen L, Conover CA, Oxvig C. Pregnancy-associated plasma protein-A2 (PAPP-A2), a novel insulin-like growth factor-binding protein-5 proteinase. J Biol Chem 2001;276:21849-53.
- Boldt HB, Conover CA. Overexpression of pregnancy-associated plasma protein-A in ovarian cancer cells promotes tumor growth in vivo. Endocrinology 2011;152:1470-8.
- Thomsen J, Hjortebjerg R, Espelund U, Ørtoft G, Vestergaard P, Magnusson NE, et al. PAPP-A proteolytic activity enhances IGF bioactivity in ascites from women with ovarian carcinoma. Oncotarget 2015;6:32266-78.
- Becker MA, Haluska P Jr., Bale LK, Oxvig C, Conover CA. A novel neutralizing antibody targeting pregnancy-associated plasma protein-a inhibits ovarian cancer growth and ascites accumulation in patient mouse tumorgrafts. Mol Cancer Ther 2015;14:973-81.
- Kalli KR, Chen BK, Bale LK, Gernand E, Overgaard MT, Oxvig C, et al. Pregnancy-associated plasma protein-A (PAPP-A) expression and insulin-like growth factor binding protein-4 protease activity in normal and malignant ovarian surface epithelial cells. Int J Cancer 2004;110:633-40.
- Lukanova A, Lundin E, Toniolo P, Micheli A, Akhmedkhanov A, Rinaldi S, et al. Circulating levels of insulin-like growth factor-I and risk of ovarian cancer. Int J Cancer 2002;101:549-54.
- Peeters PH, Lukanova A, Allen N, Berrino F, Key T, Dossus L, et al. Serum IGF-I, its major binding protein (IGFBP-3) and epithelial ovarian cancer risk: The European Prospective Investigation into Cancer and Nutrition (EPIC). Endocr Relat Cancer 2007;14:81-90.
- King SM, Modi DA, Eddie SL, Burdette JE. Insulin and insulin-like growth factor signaling increases proliferation and hyperplasia of the ovarian surface epithelium and decreases follicular integrity through upregulation of the PI3-kinase pathway. J Ovarian Res 2013;6:12.
- Sasaki H, Hayakawa J, Terai Y, Kanemura M, Tanabe-Kimura A, Kamegai H, et al. Difference between genomic actions of estrogen versus raloxifene in human ovarian cancer cell lines. Oncogene 2008;27:2737-45.
- Walker G, MacLeod K, Williams AR, Cameron DA, Smyth JF, Langdon SP. Insulin-like growth factor binding proteins IGFBP3, IGFBP4, and IGFBP5 predict endocrine responsiveness in patients with ovarian cancer. Clin Cancer Res 2007;13:1438-44.
- Irie HY, Shrestha Y, Selfors LM, Frye F, Iida N, Wang Z, et al. PTK6 regulates IGF-1-induced anchorage-independent survival. PLoS One 2010;5:e11729.
- Zhang W, Zong CS, Hermanto U, Lopez-Bergami P, Ronai Z, Wang LH. RACK1 recruits STAT3 specifically to insulin and insulin-like growth factor 1 receptors for activation, which is important for regulating anchorage-independent growth. Mol Cell Biol 2006;26:413-24.
- Martin-Belmonte F, Perez-Moreno M. Epithelial cell polarity, stem cells and cancer. Nat Rev Cancer 2011;12:23-38.
- Shen X, Xi G, Maile LA, Wai C, Rosen CJ, Clemmons DR. Insulin-like growth factor (IGF) binding protein 2 functions coordinately with receptor protein tyrosine phosphatase ß and the IGF-I receptor to regulate IGF-I-stimulated signaling. Mol Cell Biol 2012;32:4116-30.
- Shen X, Xi G, Wai C, Clemmons DR. The coordinate cellular response to insulin-like growth factor-I (IGF-I) and insulin-like growth factor-binding protein-2 (IGFBP-2) is regulated through vimentin binding to receptor tyrosine phosphatase ß (RPTPß). J Biol Chem 2015;290:11578-90.
- Seto KK, Andrulis IL. Atypical protein kinase C zeta: Potential player in cell survival and cell migration of ovarian cancer. PLoS One 2015;10:e0123528.
- Berchuck A, Iversen ES, Lancaster JM, Pittman J, Luo J, Lee P, et al. Patterns of gene expression that characterize long-term survival in advanced stage serous ovarian cancers. Clin Cancer Res 2005;11:3686-96.
- Murphy SK, Huang Z, Wen Y, Spillman MA, Whitaker RS, Simel LR, et al. Frequent IGF2/H19 domain epigenetic alterations and elevated IGF2 expression in epithelial ovarian cancer. Mol Cancer Res 2006;4:283-92.
- Li T, Chen H, Li W, Cui J, Wang G, Hu X, et al. Promoter histone H3K27 methylation in the control of IGF2 imprinting in human tumor cell lines. Hum Mol Genet 2014;23:117-28.
- Foekens JA, Portengen H, Janssen M, Klijn JG. Insulin-like growth factor-1 receptors and insulin-like growth factor-1-like activity in human primary breast cancer. Cancer 1989;63:2139-47.
- Wiley A, Katsaros D, Chen H, Rigault de la Longrais IA, Beeghly A, Puopolo M, et al. Aberrant promoter methylation of multiple genes in malignant ovarian tumors and in ovarian tumors with low malignant potential. Cancer 2006;107:299-308.
- Huang YF, Cheng WF, Wu YP, Cheng YM, Hsu KF, Chou CY. Circulating IGF system and treatment outcome in epithelial ovarian cancer. Endocr Relat Cancer 2014;21:217-29.
- Tas F, Karabulut S, Serilmez M, Ciftci R, Duranyildiz D. Clinical significance of serum insulin-like growth factor-1 (IGF-1) and insulinlike growth factor binding protein-3 (IGFBP-3) in patients with epithelial ovarian cancer. Tumour Biol 2014;35:3125-32.
- Sayer RA, Lancaster JM, Pittman J, Gray J, Whitaker R, Marks JR, et al. High insulin-like growth factor-2 (IGF-2) gene expression is an independent predictor of poor survival for patients with advanced stage serous epithelial ovarian cancer. Gynecol Oncol 2005;96:355-61.
- Wang Q, Bian CE, Peng H, He L, Zhao X. Association of circulating insulin-like growth factor 1 and insulin-like growth factor binding protein 3 with the risk of ovarian cancer: A systematic review and meta-analysis. Mol Clin Oncol 2015;3:623-8.
- Levine ME, Suarez JA, Brandhorst S, Balasubramanian P, Cheng CW, Madia F, et al. Low protein intake is associated with a major reduction in IGF-1, cancer, and overall mortality in the 65 and younger but not older population. Cell Metab 2014;19:407-17.
- Fan C, Huang YX, Bao YL, Sun LG, Wu Y, Yu CL, et al. Virtual screening of specific insulin-like growth factor 1 receptor (IGF1R) inhibitors from the National Cancer Institute (NCI) molecular database. Int J Mol Sci 2012;13:17185-209.
- Janssen JA, Varewijck AJ. IGF-IR targeted therapy: Past, present and future. Front Endocrinol (Lausanne) 2014;5:224.
- Pan H, Hanada S, Zhao J, Mao L, Ma MZ. Protein secretion is required for pregnancy-associated plasma protein-A to promote lung cancer growth in vivo. PLoS One 2012;7:e48799.
- Mitsuhashi A, Kiyokawa T, Sato Y, Shozu M. Effects of metformin on endometrial cancer cell growth in vivo: A preoperative prospective trial. Cancer 2014;120:2986-95.

| Figure 1The insulin-like growth factor 1-signaling pathway in cells. Insulin-like growth factor 1 activates both phosphatidylinositol 3-kinase/Akt and Ras/mitogen-activated protein kinase pathway resulting in cell proliferation, increased protein synthesis, and increased glucose metabolism. Phosphatidylinositol 3-kinase/Akt activates nuclear factor-κB for cell survival and inhibits apoptosis through inhibition of BAD and FKHR. Insulin-like growth factor-binding proteins modulate the bioavailability of insulin-like growth factor through direct binding in the extracellular space. Insulin-like growth factor-binding proteins also exert several insulin-like growth factor-independent effects through direct interaction with cell membrane-bound proteins, such as integrins
References
- Ferlay J, Steliarova-Foucher E, Lortet-Tieulent J, Rosso S, Coebergh JW, Comber H, et al. Cancer incidence and mortality patterns in Europe: Estimates for 40 countries in 2012. Eur J Cancer 2013;49:1374-403.
- Swaminathan R, Shanta V. Population based Cancer Registry, Chennai Cancer Institute (WIA), Adyar, Chennai. Individual Registry Write-up: 2006-2008; 2010. p. 165-83.
- Kaku T, Ogawa S, Kawano Y, Ohishi Y, Kobayashi H, Hirakawa T, et al. Histological classification of ovarian cancer. Med Electron Microsc 2003;36:9-17.
- Jelovac D, Armstrong DK. Recent progress in the diagnosis and treatment of ovarian cancer. CA Cancer J Clin 2011;61:183-203.
- Weroha SJ, Haluska P. The insulin-like growth factor system in cancer. Endocrinol Metab Clin North Am 2012;41:335-50, vi.
- Forbes BE, McCarthy P, Norton RS. Insulin-like growth factor binding proteins: A structural perspective. Front Endocrinol (Lausanne) 2012;3:38.
- Oosterhuis GJ, Vermes I, Lambalk CB, Michgelsen HW, Schoemaker J. Insulin-like growth factor (IGF)-I and IGF binding protein-3 concentrations in fluid from human stimulated follicles. Hum Reprod 1998;13:285-9.
- Brokaw J, Katsaros D, Wiley A, Lu L, Su D, Sochirca O, et al. IGF-I in epithelial ovarian cancer and its role in disease progression. Growth Factors 2007;25:346-54.
- Douglas JB, Silverman DT, Pollak MN, Tao Y, Soliman AS, Stolzenberg-Solomon RZ. Serum IGF-I, IGF-II, IGFBP-3, and IGF-I/IGFBP-3 molar ratio and risk of pancreatic cancer in the prostate, lung, colorectal, and ovarian cancer screening trial. Cancer Epidemiol Biomarkers Prev 2010;19:2298-306.
- Burger AM, Leyland-Jones B, Banerjee K, Spyropoulos DD, Seth AK. Essential roles of IGFBP-3 and IGFBP-rP1 in breast cancer. Eur J Cancer 2005;41:1515-27.
- Conover CA, Hartmann LC, Bradley S, Stalboerger P, Klee GG, Kalli KR, et al. Biological characterization of human epithelial ovarian carcinoma cells in primary culture: The insulin-like growth factor system. Exp Cell Res 1998;238:439-49.
- Cheng AJ, Chen LC, Chien KY, Chen YJ, Chang JT, Wang HM, et al. Oral cancer plasma tumor marker identified with bead-based affinity-fractionated proteomic technology. Clin Chem 2005;51:2236-44.
- Marks AG, Carroll JM, Purnell JQ, Roberts CT Jr. Plasma distribution and signaling activities of IGF-II precursors. Endocrinology 2011;152:922-30.
- Kennedy SG, Wagner AJ, Conzen SD, Jordán J, Bellacosa A, Tsichlis PN, et al. The PI 3-kinase/Akt signaling pathway delivers an anti-apoptotic signal. Genes Dev 1997;11:701-13.
- Pouysségur J, Volmat V, Lenormand P. Fidelity and spatio-temporal control in MAP kinase (ERKs) signalling. Biochem Pharmacol 2002;64:755-63.
- Han J, Jogie-Brahim S, Harada A, Oh Y. Insulin-like growth factor-binding protein-3 suppresses tumor growth via activation of caspase-dependent apoptosis and cross-talk with NF-κB signaling. Cancer Lett 2011;307:200-10.
- Peruzzi F, Prisco M, Dews M, Salomoni P, Grassilli E, Romano G, et al. Multiple signaling pathways of the insulin-like growth factor 1 receptor in protection from apoptosis. Mol Cell Biol 1999;19:7203-15.
- Galvan V, Logvinova A, Sperandio S, Ichijo H, Bredesen DE. Type 1 insulin-like growth factor receptor (IGF-IR) signaling inhibits apoptosis signal-regulating kinase 1 (ASK1). J Biol Chem 2003;278:13325-32.
- Yao JE, Yan M, Guan Z, Pan CB, Xia LP, Li CX, et al. Aurora-A down-regulates IkappaBalpha via Akt activation and interacts with insulin-like growth factor-1 induced phosphatidylinositol 3-kinase pathway for cancer cell survival. Mol Cancer 2009;8:95.
- Shao M, Hollar S, Chambliss D, Schmitt J, Emerson R, Chelladurai B, et al. Targeting the insulin growth factor and the vascular endothelial growth factor pathways in ovarian cancer. Mol Cancer Ther 2012;11:1576-86.
- Rho SB, Dong SM, Kang S, Seo SS, Yoo CW, Lee DO, et al. Insulin-like growth factor-binding protein-5 (IGFBP-5) acts as a tumor suppressor by inhibiting angiogenesis. Carcinogenesis 2008;29:2106-11.
- Baron-Hay S, Boyle F, Ferrier A, Scott C. Elevated serum insulin-like growth factor binding protein-2 as a prognostic marker in patients with ovarian cancer. Clin Cancer Res 2004;10:1796-806.
- Wolin KY, Carson K, Colditz GA. Obesity and cancer. Oncologist 2010;15:556-65.
- Farghaly SA. Ovarian cancer in obese women: Risk and optimal medical and surgical treatment options. Womens Health (Lond) 2015;11:261-3.
- Rose DP, Komninou D, Stephenson GD. Obesity, adipocytokines, and insulin resistance in breast cancer. Obes Rev 2004;5:153-65.
- Benedetto C, Salvagno F, Canuto EM, Gennarelli G. Obesity and female malignancies. Best Pract Res Clin Obstet Gynaecol 2015;29:528-40.
- Gunter MJ, Hoover DR, Yu H, Wassertheil-Smoller S, Manson JE, Li J, et al. Aprospective evaluation of insulin and insulin-like growth factor-I as risk factors for endometrial cancer. Cancer Epidemiol Biomarkers Prev 2008;17:921-9.
- Endogenous Hormones and Breast Cancer Collaborative Group, Key TJ, Appleby PN, Reeves GK, Roddam AW. Insulin-like growth factor 1 (IGF1), IGF binding protein 3 (IGFBP3), and breast cancer risk: Pooled individual data analysis of 17 prospective studies. Lancet Oncol 2010;11:530-42.
- Shimasaki S, Ling N. Identification and molecular characterization of insulin-like growth factor binding proteins (IGFBP-1, -2, -3, -4, -5 and -6). Prog Growth Factor Res 1991;3:243-66.
- Hofmann J, Wegmann B, Hackenberg R, Kunzmann R, Schulz KD, Havemann K. Production of insulin-like growth factor binding proteins by human ovarian carcinoma cells. J Cancer Res Clin Oncol 1994;120:137-42.
- Lin B, White JT, Wu J, Lele S, Old LJ, Hood L, et al. Deep depletion of abundant serum proteins reveals low-abundant proteins as potential biomarkers for human ovarian cancer. Proteomics Clin Appl 2009;3:853-61.
- Fowler DJ, Nicolaides KH, Miell JP. Insulin-like growth factor binding protein-1 (IGFBP-1): A multifunctional role in the human female reproductive tract. Hum Reprod Update 2000;6:495-504.
- Sugita S, Morishita Y, Kano J, Furuya S, Shiba-Ishii A, Noguchi M. IGFBP-1 is expressed specifically in ovarian clear cell adenocarcinoma. Histopathology 2011;58:729-38.
- Lee EJ, Mircean C, Shmulevich I, Wang H, Liu J, Niemistö A, et al. Insulin-like growth factor binding protein 2 promotes ovarian cancer cell invasion. Mol Cancer 2005;4:7.
- Flyvbjerg A, Mogensen O, Mogensen B, Nielsen OS. Elevated serum insulin-like growth factor-binding protein 2 (IGFBP-2) and decreased IGFBP-3 in epithelial ovarian cancer: Correlation with cancer antigen 125 and tumor-associated trypsin inhibitor. J Clin Endocrinol Metab 1997;82:2308-13.
- Wang H, Rosen DG, Wang H, Fuller GN, Zhang W, Liu J. Insulin-like growth factor-binding protein 2 and 5 are differentially regulated in ovarian cancer of different histologic types. Mod Pathol 2006;19:1149-56.
- Karasik A, Menczer J, Pariente C, Kanety H. Insulin-like growth factor-I (IGF-I) and IGF-binding protein-2 are increased in cyst fluids of epithelial ovarian cancer. J Clin Endocrinol Metab 1994;78:271-6.
- Kanety H, Kattan M, Goldberg I, Kopolovic J, Ravia J, Menczer J, et al. Increased insulin-like growth factor binding protein-2 (IGFBP-2) gene expression and protein production lead to high IGFBP-2 content in malignant ovarian cyst fluid. Br J Cancer 1996;73:1069-73.
- Torng PL, Lin CW, Chan MW, Yang HW, Huang SC, Lin CT. Promoter methylation of IGFBP-3 and p53 expression in ovarian endometrioid carcinoma. Mol Cancer 2009;8:120.
- Chen X, Ferry RJ Jr. Novel actions of IGFBP-3 on intracellular signaling pathways of insulin-secreting cells. Growth Horm IGF Res 2006;16:41-8.
- Tonin P, Ehrenborg E, Lenoir G, Feunteun J, Lynch H, Morgan K, et al. The human insulin-like growth factor-binding protein 4 gene maps to chromosome region 17q12-q21.1 and is close to the gene for hereditary breast-ovarian cancer. Genomics 1993;18:414-7.
- Durai R, Davies M, Yang W, Yang SY, Seifalian A, Goldspink G, et al. Biology of insulin-like growth factor binding protein-4 and its role in cancer (review). Int J Oncol 2006;28:1317-25.
- Dikshit A, Gao C, Small C, Hales K, Hales DB. Flaxseed and its components differentially affect estrogen targets in pre-neoplastic hen ovaries. J Steroid Biochem Mol Biol 2016;159:73-85.
- Mosig RA, Lobl M, Senturk E, Shah H, Cohen S, Chudin E, et al. IGFBP-4 tumor and serum levels are increased across all stages of epithelial ovarian cancer. J Ovarian Res 2012;5:3.
- ;Zhu W, Shiojima I, Ito Y, Li Z, Ikeda H, Yoshida M, et al. IGFBP-4 is an inhibitor of canonical Wnt signalling required for cardiogenesis. Nature 2008;454:345-9.
- Zhang C, Lu L, Li Y, Wang X, Zhou J, Liu Y, et al. IGF binding protein-6 expression in vascular endothelial cells is induced by hypoxia and plays a negative role in tumor angiogenesis. Int J Cancer 2012;130:2003-12.
- Yang Z, Bach LA. Differential effects of insulin-like growth factor binding protein-6 (IGFBP-6) on migration of two ovarian cancer cell lines. Front Endocrinol (Lausanne) 2015;5:231.
- Bahrani-Mostafavi Z, Tickle TL, Zhang J, Bennett KE, Vachris JC, Spencer MD, et al. Correlation analysis of HOX, ErbB and IGFBP family gene expression in ovarian cancer. Cancer Invest 2008;26:990-8.
- Gunawardana CG, Kuk C, Smith CR, Batruch I, Soosaipillai A, Diamandis EP. Comprehensive analysis of conditioned media from ovarian cancer cell lines identifies novel candidate markers of epithelial ovarian cancer. J Proteome Res 2009;8:4705-13.
- Lin TM, Halbert SP, Spellacy WN. Measurement of pregnancy-associated plasma proteins during human gestation. J Clin Invest 1974;54:576-82.
- Conover CA, Oxvig C, Overgaard MT, Christiansen M, Giudice LC. Evidence that the insulin-like growth factor binding protein-4 protease in human ovarian follicular fluid is pregnancy associated plasma protein-A. J Clin Endocrinol Metab 1999;84:4742-5.
- Mazerbourg S, Overgaard MT, Oxvig C, Christiansen M, Conover CA, Laurendeau I, et al. Pregnancy-associated plasma protein-A (PAPP-A) in ovine, bovine, porcine, and equine ovarian follicles: Involvement in IGF binding protein-4 proteolytic degradation and mRNA expression during follicular development. Endocrinology 2001;142:5243-53.
- Hourvitz A, Kuwahara A, Hennebold JD, Tavares AB, Negishi H, Lee TH, et al. The regulated expression of the pregnancy-associated plasma protein-A in the rodent ovary: A proposed role in the development of dominant follicles and of corpora lutea. Endocrinology 2002;143:1833-44.
- Juengel JL, Haydon LJ, Mester B, Thomson BP, Beaumont M, Eckery DC. The role of IGFs in the regulation of ovarian follicular growth in the brushtail possum (Trichosurus vulpecula). Reproduction 2010;140:295-303.
- Mazerbourg S, Bondy CA, Zhou J, Monget P. The insulin-like growth factor system: A key determinant role in the growth and selection of ovarian follicles? a comparative species study. Reprod Domest Anim 2003;38:247-58.
- Spicer LJ. Proteolytic degradation of insulin-like growth factor binding proteins by ovarian follicles: A control mechanism for selection of dominant follicles. Biol Reprod 2004;70:1223-30.
- Laursen LS, Overgaard MT, Weyer K, Boldt HB, Ebbesen P, Christiansen M, et al. Cell surface targeting of pregnancy-associated plasma protein A proteolytic activity. Reversible adhesion is mediated by two neighboring short consensus repeats. J Biol Chem 2002;277:47225-34.
- Laursen LS, Kjaer-Sorensen K, Andersen MH, Oxvig C. Regulation of insulin-like growth factor (IGF) bioactivity by sequential proteolytic cleavage of IGF binding protein-4 and -5. Mol Endocrinol 2007;21:1246-57.
- Monget P, Mazerbourg S, Delpuech T, Maurel MC, Manière S, Zapf J, et al. Pregnancy-associated plasma protein-A is involved in insulin-like growth factor binding protein-2 (IGFBP-2) proteolytic degradation in bovine and porcine preovulatory follicles: Identification of cleavage site and characterization of IGFBP-2 degradation. Biol Reprod 2003;68:77-86.
- Laursen LS, Overgaard MT, Søe R, Boldt HB, Sottrup-Jensen L, Giudice LC, et al. Pregnancy-associated plasma protein-A (PAPP-A) cleaves insulin-like growth factor binding protein (IGFBP)-5 independent of IGF: Implications for the mechanism of IGFBP-4 proteolysis by PAPP-A. FEBS Lett 2001;504:36-40.
- Ning Y, Schuller AG, Conover CA, Pintar JE. Insulin-like growth factor (IGF) binding protein-4 is both a positive and negative regulator of IGF activity in vivo. Mol Endocrinol 2008;22:1213-25.
- Overgaard MT, Boldt HB, Laursen LS, Sottrup-Jensen L, Conover CA, Oxvig C. Pregnancy-associated plasma protein-A2 (PAPP-A2), a novel insulin-like growth factor-binding protein-5 proteinase. J Biol Chem 2001;276:21849-53.
- Boldt HB, Conover CA. Overexpression of pregnancy-associated plasma protein-A in ovarian cancer cells promotes tumor growth in vivo. Endocrinology 2011;152:1470-8.
- Thomsen J, Hjortebjerg R, Espelund U, Ørtoft G, Vestergaard P, Magnusson NE, et al. PAPP-A proteolytic activity enhances IGF bioactivity in ascites from women with ovarian carcinoma. Oncotarget 2015;6:32266-78.
- Becker MA, Haluska P Jr., Bale LK, Oxvig C, Conover CA. A novel neutralizing antibody targeting pregnancy-associated plasma protein-a inhibits ovarian cancer growth and ascites accumulation in patient mouse tumorgrafts. Mol Cancer Ther 2015;14:973-81.
- Kalli KR, Chen BK, Bale LK, Gernand E, Overgaard MT, Oxvig C, et al. Pregnancy-associated plasma protein-A (PAPP-A) expression and insulin-like growth factor binding protein-4 protease activity in normal and malignant ovarian surface epithelial cells. Int J Cancer 2004;110:633-40.
- Lukanova A, Lundin E, Toniolo P, Micheli A, Akhmedkhanov A, Rinaldi S, et al. Circulating levels of insulin-like growth factor-I and risk of ovarian cancer. Int J Cancer 2002;101:549-54.
- Peeters PH, Lukanova A, Allen N, Berrino F, Key T, Dossus L, et al. Serum IGF-I, its major binding protein (IGFBP-3) and epithelial ovarian cancer risk: The European Prospective Investigation into Cancer and Nutrition (EPIC). Endocr Relat Cancer 2007;14:81-90.
- King SM, Modi DA, Eddie SL, Burdette JE. Insulin and insulin-like growth factor signaling increases proliferation and hyperplasia of the ovarian surface epithelium and decreases follicular integrity through upregulation of the PI3-kinase pathway. J Ovarian Res 2013;6:12.
- Sasaki H, Hayakawa J, Terai Y, Kanemura M, Tanabe-Kimura A, Kamegai H, et al. Difference between genomic actions of estrogen versus raloxifene in human ovarian cancer cell lines. Oncogene 2008;27:2737-45.
- Walker G, MacLeod K, Williams AR, Cameron DA, Smyth JF, Langdon SP. Insulin-like growth factor binding proteins IGFBP3, IGFBP4, and IGFBP5 predict endocrine responsiveness in patients with ovarian cancer. Clin Cancer Res 2007;13:1438-44.
- Irie HY, Shrestha Y, Selfors LM, Frye F, Iida N, Wang Z, et al. PTK6 regulates IGF-1-induced anchorage-independent survival. PLoS One 2010;5:e11729.
- Zhang W, Zong CS, Hermanto U, Lopez-Bergami P, Ronai Z, Wang LH. RACK1 recruits STAT3 specifically to insulin and insulin-like growth factor 1 receptors for activation, which is important for regulating anchorage-independent growth. Mol Cell Biol 2006;26:413-24.
- Martin-Belmonte F, Perez-Moreno M. Epithelial cell polarity, stem cells and cancer. Nat Rev Cancer 2011;12:23-38.
- Shen X, Xi G, Maile LA, Wai C, Rosen CJ, Clemmons DR. Insulin-like growth factor (IGF) binding protein 2 functions coordinately with receptor protein tyrosine phosphatase ß and the IGF-I receptor to regulate IGF-I-stimulated signaling. Mol Cell Biol 2012;32:4116-30.
- Shen X, Xi G, Wai C, Clemmons DR. The coordinate cellular response to insulin-like growth factor-I (IGF-I) and insulin-like growth factor-binding protein-2 (IGFBP-2) is regulated through vimentin binding to receptor tyrosine phosphatase ß (RPTPß). J Biol Chem 2015;290:11578-90.
- Seto KK, Andrulis IL. Atypical protein kinase C zeta: Potential player in cell survival and cell migration of ovarian cancer. PLoS One 2015;10:e0123528.
- Berchuck A, Iversen ES, Lancaster JM, Pittman J, Luo J, Lee P, et al. Patterns of gene expression that characterize long-term survival in advanced stage serous ovarian cancers. Clin Cancer Res 2005;11:3686-96.
- Murphy SK, Huang Z, Wen Y, Spillman MA, Whitaker RS, Simel LR, et al. Frequent IGF2/H19 domain epigenetic alterations and elevated IGF2 expression in epithelial ovarian cancer. Mol Cancer Res 2006;4:283-92.
- Li T, Chen H, Li W, Cui J, Wang G, Hu X, et al. Promoter histone H3K27 methylation in the control of IGF2 imprinting in human tumor cell lines. Hum Mol Genet 2014;23:117-28.
- Foekens JA, Portengen H, Janssen M, Klijn JG. Insulin-like growth factor-1 receptors and insulin-like growth factor-1-like activity in human primary breast cancer. Cancer 1989;63:2139-47.
- Wiley A, Katsaros D, Chen H, Rigault de la Longrais IA, Beeghly A, Puopolo M, et al. Aberrant promoter methylation of multiple genes in malignant ovarian tumors and in ovarian tumors with low malignant potential. Cancer 2006;107:299-308.
- Huang YF, Cheng WF, Wu YP, Cheng YM, Hsu KF, Chou CY. Circulating IGF system and treatment outcome in epithelial ovarian cancer. Endocr Relat Cancer 2014;21:217-29.
- Tas F, Karabulut S, Serilmez M, Ciftci R, Duranyildiz D. Clinical significance of serum insulin-like growth factor-1 (IGF-1) and insulinlike growth factor binding protein-3 (IGFBP-3) in patients with epithelial ovarian cancer. Tumour Biol 2014;35:3125-32.
- Sayer RA, Lancaster JM, Pittman J, Gray J, Whitaker R, Marks JR, et al. High insulin-like growth factor-2 (IGF-2) gene expression is an independent predictor of poor survival for patients with advanced stage serous epithelial ovarian cancer. Gynecol Oncol 2005;96:355-61.
- Wang Q, Bian CE, Peng H, He L, Zhao X. Association of circulating insulin-like growth factor 1 and insulin-like growth factor binding protein 3 with the risk of ovarian cancer: A systematic review and meta-analysis. Mol Clin Oncol 2015;3:623-8.
- Levine ME, Suarez JA, Brandhorst S, Balasubramanian P, Cheng CW, Madia F, et al. Low protein intake is associated with a major reduction in IGF-1, cancer, and overall mortality in the 65 and younger but not older population. Cell Metab 2014;19:407-17.
- Fan C, Huang YX, Bao YL, Sun LG, Wu Y, Yu CL, et al. Virtual screening of specific insulin-like growth factor 1 receptor (IGF1R) inhibitors from the National Cancer Institute (NCI) molecular database. Int J Mol Sci 2012;13:17185-209.
- Janssen JA, Varewijck AJ. IGF-IR targeted therapy: Past, present and future. Front Endocrinol (Lausanne) 2014;5:224.
- Pan H, Hanada S, Zhao J, Mao L, Ma MZ. Protein secretion is required for pregnancy-associated plasma protein-A to promote lung cancer growth in vivo. PLoS One 2012;7:e48799.
- Mitsuhashi A, Kiyokawa T, Sato Y, Shozu M. Effects of metformin on endometrial cancer cell growth in vivo: A preoperative prospective trial. Cancer 2014;120:2986-95.


 PDF
PDF  Views
Views  Share
Share

