Should every patient with pancreatic cancer receive perioperative/neoadjuvant therapy?
CC BY-NC-ND 4.0 · Indian J Med Paediatr Oncol 2016; 37(04): 211-213
DOI: DOI: 10.4103/0971-5851.195731
Abstract
Pancreatic ductal adenocarcinoma is a highly aggressive disease, and medical as well as surgical therapeutic options are limited. This article reviews stage dependent treatment options, with a special focus on the current controversy of perioperative treatment regimens in initially borderline resectable or locally advanced patients. Neoadjuvant treatment can potentially increase the rate of complete tumor resection and may be more effective than adjuvant systemic therapy. Further, in the case of disease progression during or after neoadjuvant therapy, patients can be spared extensive surgery. Today, common therapeutic regimens include gemcitabine/nab-paclitaxel and FOLFIRINOX, as well as chemoradiation. However, because of the paucity of evidence from randomized trials, most guidelines do not recommend neoadjuvant therapy in resectable tumors, and for borderline or locally advanced tumors only within clinical trials. Importantly, every patient should be discussed in multidisciplinary tumor boards.
Publication History
Article published online:
12 July 2021
© 2016. Indian Society of Medical and Paediatric Oncology. This is an open access article published by Thieme under the terms of the Creative Commons Attribution-NonDerivative-NonCommercial-License, permitting copying and reproduction so long as the original work is given appropriate credit. Contents may not be used for commercial purposes, or adapted, remixed, transformed or built upon. (https://creativecommons.org/licenses/by-nc-nd/4.0/.)
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India
Abstract
Pancreatic ductal adenocarcinoma is a highly aggressive disease, and medical as well as surgical therapeutic options are limited. This article reviews stage dependent treatment options, with a special focus on the current controversy of perioperative treatment regimens in initially borderline resectable or locally advanced patients. Neoadjuvant treatment can potentially increase the rate of complete tumor resection and may be more effective than adjuvant systemic therapy. Further, in the case of disease progression during or after neoadjuvant therapy, patients can be spared extensive surgery. Today, common therapeutic regimens include gemcitabine/nab-paclitaxel and FOLFIRINOX, as well as chemoradiation. However, because of the paucity of evidence from randomized trials, most guidelines do not recommend neoadjuvant therapy in resectable tumors, and for borderline or locally advanced tumors only within clinical trials. Importantly, every patient should be discussed in multidisciplinary tumor boards.
INTRODUCTION
Pancreatic ductal adenocarcinoma (PDAC) is associated with the worst prognosis of all gastrointestinal malignancies, mainly due to late and unspecific symptoms, an aggressive tumor biology, and resistance to most therapies.[1] Only 10–20% of patients are resectable at presentation.[1] For many years, these patients were subjected to primary resection; however, new data suggest that this group of patients may benefit from perioperative/neoadjuvant systemic chemotherapy.[2] Another 30–40% of patients present with borderline resectable or locally advanced/unresectable tumors.[3] Here, the risk of occult distant metastasis is higher than in patients with smaller resectable tumors, and perioperative/neoadjuvant treatment is generally recommended to downsize the primary tumor and estimate tumor biology.[4,5] Depending on the patient's performance status and preferences, more or less aggressive regimens can be chosen.[4] The further course is determined to be neoadjuvant/curative or palliative depending on the radiological and tumor marker response. However, radiological response prediction is difficult, and tumor markers are informative only in a subgroup of patients. The largest group of patients (50%) with PDAC has metastases at the time of diagnosis which is associated with a very limited prognosis of < 1 year. For these patients, interdisciplinary determined palliative treatment adapted to the physical condition and patients’ preferences is indicated.[6,7] This review discusses the current controversy whether patients with PDAC should consistently be treated in a perioperative/neoadjuvant setting. Further, different treatment approaches for patients with PDAC who are resectable or can likely be converted to resectability are briefly addressed.
TREATMENT RESPONSE AND SURGICAL RESECTABILITY
Accurate staging and discussion in multidisciplinary tumor boards are necessary to classify the extent of tumor growth/tumor stage and to be able to make treatment decisions. Classification of resectability into borderline resectable and locally advanced, unresectable disease should follow the most widely used radiological guidelines of the National Comprehensive Cancer Network,[5] which have also been adopted by the International Study Group for Pancreatic Surgery.[8] However, re-classification during or after (neoadjuvant) treatment has turned out to be difficult as radiological findings are often not able to discriminate between vital tumor cells and fibrotic nonmalignant stroma, which may result from effective neoadjuvant treatment.[3,9] Thus, if in doubt, liberal indication for surgical exploration can be beneficial for those patients with radiological unclear response. Indeed, some centers advocate exploration in all patients whose tumors do not progress during therapy.[9]
FROM ADJUVANT TO NEOADJUVANT TREATMENT IN PANCREATIC DUCTAL ADENOCARCINOMA
Depending on the tumor localization and the grade of vessel infiltration, surgical resections are highly complex. First approaches to PDACs that were resectable or borderline resectable (at least not clearly unresectable) consisted of exploration with primary tumor removal if possible.[3] However, over three-quarter of patients with any extent of the primary PDAC, who undergo macroscopic complete surgical resection will develop distant metastases as the first evidence of recurrence.[4] Thus, clinically occult metastases may be present already at the time of diagnosis, which limits postoperative prognosis. This is one reason why adjuvant chemotherapy is effective and recommended for all patients after resection of PDAC.[3] However, due to the extent of surgery and delayed recovery and rehabilitation, in approximately 50% of all cases, adjuvant chemotherapy is not given as planned preoperatively.[4] Due to this fact, recent recommendations advocate neoadjuvant treatment not only for patients with locally advanced/borderline resectable PDAC.[4] Rather, neoadjuvant schemes are also preferred over adjuvant schemes for patients with primary resectable tumors.[4] Since there has been a lack of effective single or combination chemotherapies for PDAC, traditionally, preoperative therapy consisted of chemoradiation.[3] This resulted in an increased median postoperative survival of nearly 2 years for initial resectable patients who underwent neoadjuvant therapy.[2] Within the last two decades, many neoadjuvant treatment protocols have been developed since a higher chance of local control was shown in retrospective analyses of PDAC patients who underwent preoperative therapy.[3] However, till date, no completed randomized controlled clinical trial on the effectiveness of neoadjuvant therapy has been published.
RATIONALE OF NEOADJUVANT TREATMENT
Preoperative treatment, such as neoadjuvant chemotherapy or chemoradiation, may be an effective strategy for all patients with PDAC regardless the local tumor extent. More patients are able to complete therapy in a preoperative setting since approximately 50% of patients do not complete adjuvant therapy as planned preoperatively due to delayed recovery and rehabilitation.[4] Further, patients’ tolerance and compliance to the therapy may be increased.[9] Furthermore, neoadjuvant treatment can induce tumor regression, which may increase the likelihood of complete tumor resection (R0 resection) in both, borderline and primary resectable tumors. However, at the moment, evidence-based guidelines for neoadjuvant therapy in primary resectable pancreatic cancer are still missing due to lack of randomized studies.[5,8] At least, up to 50% of initially borderline/unresectable patients become eligible for surgical resection after neoadjuvant therapy.[2,3] Further rationale for a neoadjuvant approach for all patients with PDAC regardless of the tumor extent is that during an induction therapy of 2–3 months, unfavorable tumor biology can be anticipated if progression occurs during therapy.[4] In the subset of 20–30% of patients who develop local progression or distant metastasis under induction chemotherapy, an operation with its associated morbidity can therefore be avoided.[4] Initial concerns that the time point of resectability is missed by neoadjuvant therapy and postoperative complications are elevated have neither been proven nor ruled out.[4] In fact, disease progression during or after neoadjuvant therapy, if it occurs, is usually seen at distant sites such as the liver, peritoneum, and lung, and < 1% of patients develop isolated local disease progression at the time of restaging after neoadjuvant therapy.[4] Furthermore, the texture of a preoperative treated pancreas usually becomes more firm and has reduced enzyme production, which would be more likely to reduce the rates of pancreatic fistula and anastomotic leakage, the most frequent serious postoperative complications, rather than to increase it.[4] Even if a tumor is radiologically staged resectable from the beginning, there seems to be a higher rate of R0 resections after neoadjuvant-combined chemoradiation.[4] For all that, no differences in the postoperative mortality and morbidity could be shown.[4] However, potential disadvantages of neoadjuvant therapy are complications from pretreatment endoscopic or endosonographic interventions (i.e., fine needle aspiration or true cut biopsy) for histological proof of PDAC before therapy, as well as the challenge of collaboration between different disciplines (oncologists, pathologists, radiation oncologists, and surgeons).[4] Further, even if the primary tumor shrinks, the relationship of the tumor to the adjacent vessels may stay unchanged, and only in a small percentage, a change in the clinical tumor stage has been reported.[4] Finally, tumor progression cannot be excluded in the setting of a tumor that does not respond to neoadjuvant treatment.
TREATMENT ALGORITHM FOR PANCREATIC DUCTAL ADENOCARCINOMA
Chemotherapeutic regimens have evolved from gemcitabine alone to 5-FU, leucovorin, irinotecan, oxaliplatin (FOLFIRINOX),[7] gemcitabine, docetaxel, capecitabine (GTX), gemcitabine/nab-paclitaxel,[6] and other less established combination therapies.[4,10] Because recommendations differ, individualized concepts should be discussed for every patient in multidisciplinary tumor boards. Schemes as well as dose titration are beyond the scope of this review. A recently suggested approach is to administer 2 months of neoadjuvant chemotherapy, followed by restaging. In the case of no significant tumor regression by radiological and cancer antigen 19-9 findings, rather transition to chemoradiation to reach resectability is recommended than the second-line systemic chemotherapy.[4] Figure 1 depicts a flowchart with all currently justifiable treatment options depending on the stage and extent of PDAC.
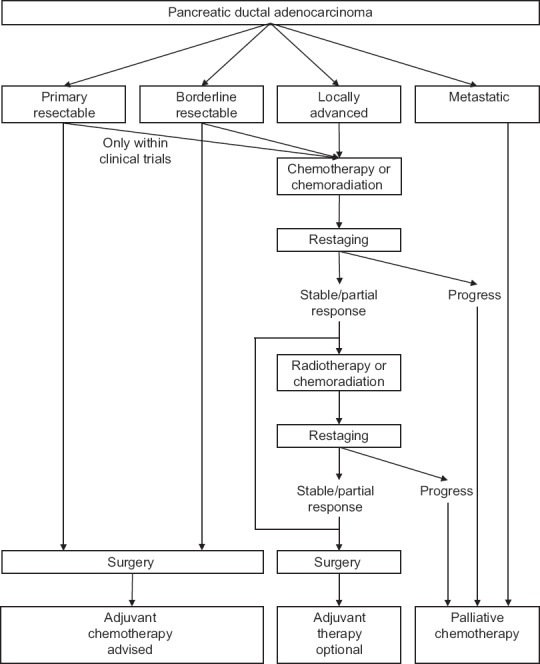
| Fig. 1 Treatment options for pancreatic ductal adenocarcinoma depending on local tumor extent and distant dissemination
SUMMARY
In contrast to many other solid tumors, the treatment algorithm for patients with localized PDAC, especially borderline resectable and locally advanced tumors, remains controversial and multimodal therapies are increasing. Due to shrinkage of the primary tumor as well as putative destruction of distant micrometastases, preliminary clinical data are pointing less toward primary resection, rather suggesting improved outcome for neoadjuvant-intended treatment for every patient with localized PDAC.[4] If chemotherapy alone is applied, gemcitabine/nab-paclitaxel and FOLFIRINOX over 2 months are the most common regimens,[4] under consideration of both, the patient's physical status and personal preferences, respectively. Chemoradiation can be used as well; however, compared to chemotherapy alone, it is more controversial and less effective in the palliative setting. In the case of chemoradiation, gemcitabine combined with external-beam radiation therapy is favored.[4] Finally, since there is no high-quality evidence available, it is still reasonable not to treat every patient with PDAC before surgery,[10] and indeed, most guidelines do not suggest neoadjuvant therapy in resectable tumors yet.[5,8] Finally, as radiological findings do not always represent the true extent of vital tumor, every patient who underwent neoadjuvant therapy and whose tumor did not progress may benefit from surgical exploration.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES

| Fig. 1 Treatment options for pancreatic ductal adenocarcinoma depending on local tumor extent and distant dissemination


 PDF
PDF  Views
Views  Share
Share

