Sinusoidal Obstruction Syndrome during Treatment for Wilms’ Tumor: A Life threatening Complication
CC BY-NC-ND 4.0 · Indian J Med Paediatr Oncol 2017; 38(04): 447-451
DOI: DOI: 10.4103/ijmpo.ijmpo_188_16
Abstract
Context: Survival rates exceed 90% in Wilms' tumor (WT). Actinomycin-D (ACT-D) which is indispensable in the management of WT is associated with the development of sinusoidal obstruction syndrome (SOS), a potentially fatalcomplication. Aims: The aim is to study the presentation, management, and outcome of SOScomplicating ACT-D administration in WT. Settings and Design: Retrospective file review conducted in a Pediatric Hematology-Oncology unit. Materials and Methods: Patients diagnosed and treated for WT from January 2012 to December 2015 were analyzed. SOS was diagnosed clinically, based on McDonalds criteria, requiring two of the following: jaundice, hepatomegaly and/or right upper quadrant pain, weight gain with or without ascites. Results: Of 104 patients treated, SOS occurred in 5 (4.8%). Age: 6 months to 5 years, 3 were girls. Tumor involved left kidney in 3, right in 1 and a horseshoe kidney in 1. Histopathology was consistent with WT in 4 and clear cell sarcoma kidney in 1. One had pulmonary metastases. Three developed SOS preoperatively and two during adjuvant chemotherapy. None received radiotherapy. Clinical manifestationscomprised of jaundice, hepatomegaly, ascites/weight gain, respiratory distress, hypotension, and encephalopathy. Laboratory findings included thrombocytopenia, elevated serum transaminases, and coagulopathy. Treatment included fluid restriction, broad spectrum antibiotics, and transfusional support. Two children received N-acetyl cysteine infusion. Defibrotide was administered to two patients. Four recovered and one succumbed to multi-organ failure. Two patients were safely re-challenged with 50% doses of ACT-D. Conclusions: SOS is a clinical diagnosis. Systematic supportive care can enablecomplete recovery. Under close monitoring, re-challenge of ACT-D can be performed in gradually escalating doses.
Keywords
Developing country - liver failure - portal hypertension - renal malignancy - veno-occlusive diseasePublication History
Article published online:
04 July 2021
© 2017. Indian Society of Medical and Paediatric Oncology. This is an open access article published by Thieme under the terms of the Creative Commons Attribution-NonDerivative-NonCommercial-License, permitting copying and reproduction so long as the original work is given appropriate credit. Contents may not be used forcommercial purposes, or adapted, remixed, transformed or built upon. (https://creativecommons.org/licenses/by-nc-nd/4.0/.)
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India
Abstract
Context:
Survival rates exceed 90% in Wilms' tumor (WT). Actinomycin-D (ACT-D) which is indispensable in the management of WT is associated with the development of sinusoidal obstruction syndrome (SOS), a potentially fatal complication.
Aims:
The aim is to study the presentation, management, and outcome of SOS complicating ACT-D administration in WT.
Settings and Design:
Retrospective file review conducted in a Pediatric Hematology-Oncology unit.
Materials and Methods:
Patients diagnosed and treated for WT from January 2012 to December 2015 were analyzed. SOS was diagnosed clinically, based on McDonalds criteria, requiring two of the following: jaundice, hepatomegaly and/or right upper quadrant pain, weight gain with or without ascites.
Results:
Of 104 patients treated, SOS occurred in 5 (4.8%). Age: 6 months to 5 years, 3 were girls. Tumor involved left kidney in 3, right in 1 and a horseshoe kidney in 1. Histopathology was consistent with WT in 4 and clear cell sarcoma kidney in 1. One had pulmonary metastases. Three developed SOS preoperatively and two during adjuvant chemotherapy. None received radiotherapy. Clinical manifestations comprised of jaundice, hepatomegaly, ascites/weight gain, respiratory distress, hypotension, and encephalopathy. Laboratory findings included thrombocytopenia, elevated serum transaminases, and coagulopathy. Treatment included fluid restriction, broad spectrum antibiotics, and transfusional support. Two children received N-acetyl cysteine infusion. Defibrotide was administered to two patients. Four recovered and one succumbed to multi-organ failure. Two patients were safely re-challenged with 50% doses of ACT-D.
Conclusions:
SOS is a clinical diagnosis. Systematic supportive care can enable complete recovery. Under close monitoring, re-challenge of ACT-D can be performed in gradually escalating doses.
Introduction
Contemporary 5-year survival rates exceed 90% in Wilms' tumor (WT).[1] Multidisciplinary treatment comprises of surgery and chemotherapy in all patients; and radiotherapy in Stage 3 and 4 disease.[1] Actinomycin-D (Act-D) is an integral part of chemotherapy, across all international protocols employed in WT.[1,2] Sinusoidal obstruction syndrome (SOS), is a well-known serious complication of hematopoietic stem cell transplant (HSCT).[3] However, chemotherapeutic agents such as Act-D as well as radiation can damage small hepatic venules and sinusoids, thus recapitulating SOS in nonHSCT patients.[4,5] The incidence of SOS ranges from 1.2% to 8% in children treated for WT.[4] It is also documented in other childhood malignancies such as rhabdomyosarcoma and medulloblastoma requiring Act-D as part of therapy.[6,7,8] Access to defibrotide therapy, which is utilized in SOS following HSCT, is often limited by availability and high cost in low-/middle-income countries.[3] Best possible supportive care can still offer a good chance of salvaging children with SOS.[5] This study describes a single center experience of diagnosing and managing SOS in children with WT with the feasibility of re-exposure to Act-D subsequent to complete recovery from SOS.
Materials and Methods
The study was conducted as a file review. All patients diagnosed and treated for WT/clear cell sarcoma of the kidney (CCSK) from January 2012 to December 2015 were included in the study. We treat patients as per the International Society of Paediatric Oncology Wilms' Tumour protocol (SIOP-WT2001).[2] Act-D is administered as a single dose slow intravenous bolus of 45 μg/kg. The dose was reduced to two-thirds in children weighing <12 href="https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5759062/#ref9" rid="ref9" class=" bibr popnode tag_hotlink tag_tooltip" id="__tag_659252906" role="button" aria-expanded="false" aria-haspopup="true" xss=removed>9] This required the presence of at least two of the following symptoms or signs: jaundice (serum total bilirubin >2 mg/dL), hepatomegaly and/or abdominal pain in the right upper quadrant, and 5% weight gain from baseline with or without ascites. A complete blood count, liver function tests [total serum bilirubin, direct serum bilirubin, serum aspartate aminotransferase (AST), serum alanine aminotransferase (ALT), and serum albumin] and renal function tests (blood urea nitrogen and serum creatinine) and a coagulation profile (prothrombin time, activated partial thromboplastin time, and international normalized ratio) were done in all patients suspected to have SOS. Viral markers for hepatitis B virus surface antigen, anti-hepatitis C virus IgM, anti-hepatitis A virus IgM and anti-hepatitis E virus IgM were also done in all patients. Ultrasound abdomen with Doppler for portal venous blood flow was performed in all patients.
Results
Over a period of 3 years, 104 children were diagnosed with WT/CCSK (6 being CCSK). Five (4.8%) developed SOS while on therapy. Mean age was 3.3 years (range: 6 months to 5 years). Male to female ratio was 1.5:1. Tumors were left-sided in 3, right-sided in 1 and 1 patient had tumor arising from a horseshoe kidney. Staging: one patient had stage 4 disease, with metastases to the lungs; two stage 2; one stage 1 and one patient with non-metastatic disease (histopathological staging not known as he expired before surgery). Four patients received neoadjuvant chemotherapy, with one patient undergoing a primary nephrectomy at another hospital. Features of SOS developed during neoadjuvant chemotherapy in 3, and during adjuvant therapy in 2 patients. The median duration between the start of therapy and diagnosis of SOS was 6 weeks (range: 4–13). A median number of doses of Act-D received before developing SOS was 2 (range: 2–6). None of the patients received radiation.
All patients had tender hepatomegaly and evidence of fluid accumulation (weight gain/ascites). Clinical jaundice, fever and recurrent vomiting were seen in 4. Central nervous system involvement in the form of drowsiness and altered sensorium was observed in 3. Respiratory distress with bilateral infiltrates on chest x-ray requiring oxygen support/ventilation ensued in 3, hypotensive shock necessitating inotropic support occurred in 3 and 1 patient developed acute renal failure requiring dialysis (clinical profile of patients summarized in Table 1). All patients had significant thrombocytopenia and prolonged prothrombin time and activated partial thromboplastin time. Four patients each had conjugated hyperbilirubinemia, hypoalbuminemia, and serum transaminase elevated to above 5 times of the upper limit of normal [Table 2]. Ultrasound abdomen with Doppler assessment of portal and hepatic venous blood flow was performed in all patients. While four patients demonstrated evidence of mild to moderate ascites, reversed or attenuated venous blood flow was not perceived in any. Serology for viral hepatitis was negative in all patients.
Table 1
Clinical features, treatment and outcome of patients diagnosed with sinusoidal obstruction syndrome
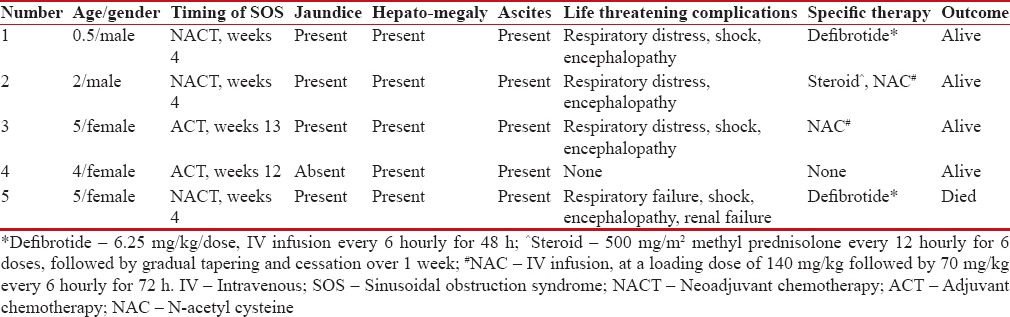
|
Table 2
Salient laboratory investigations in patients clinically diagnosed with sinusoidal obstruction syndrome (n=5)
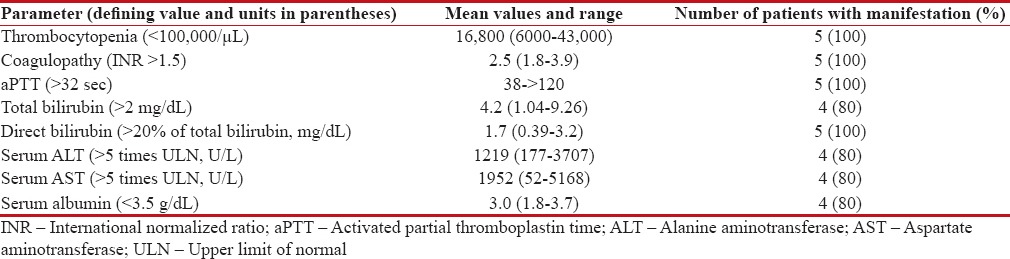
|
Treatment was mainly supportive. Patients received restricted fluids at 80% of the total daily maintenance calculated by Holliday–Segar's formula. Strict input/output records were maintained. Furosemide at a dose of 0.5–1 mg/kg was administered every 8 to 12 hourly. Platelets and fresh frozen plasma were transfused for thrombocytopenia and coagulopathy. Broad spectrum antibiotics were administered to cover possible co-existing/super added infections. Albumin was infused in patients with serum albumin <2 xss=removed>2 every 12 hourly for 6 doses, followed by gradual tapering and cessation over 1 week. Defibrotide has very limited availability in our country and is extremely expensive. Two of our patients received defibrotide at a dose of 6.25 mg/kg IV every 6 hourly, for 48 h. Two patients in addition received oral ursodeoxycholic acid 20–30 mg/kg/day in twice a day doses.
One patient succumbed to SOS with multi-organ failure. There was a complete recovery in the other 4 patients within a median duration of 10 days (range: 4–26) from diagnosis of SOS. Two of them were re-exposed to Act-D, initially at 50% dose and gradually escalated to 100% while closely monitoring for recurrence of SOS. Act-D was replaced by doxorubicin in one patient at the discretion of the treating physician. The fifth patient (CCSK) did not require further Act-D as part of his high-risk adjuvant chemotherapy protocol.
Discussion
WT is a highly curable malignancy which requires relatively less intensive chemotherapy as compared to other childhood malignancies.[1] It is indeed unfortunate when children with WT develop life-threatening complications secondary to therapy. SOS or veno-occlusive disease is classically described as an early complication of HSCT, which follows injury and narrowing of terminal hepatic venules and sinusoids.[3] A cascade of hypercoagulable and proinflammatory pathways causes further damage, resulting in obstruction of hepatic venous outflow, portal hypertension, and multi-organ failure.[3] Act-D in isolation, or in combination with hepatic radiation can potentially damage the vascular endothelium and trigger SOS in children with WT.[4,5] In the SIOP-WT2001 trial a 3% incidence of SOS was observed among 583 patients with stage II/III intermediate risk histology WT.[10] The German Paediatric Oncology and Haematology Society reported a similar incidence of 4.8% in 481 patients with WT, while the United Kingdom Children's Cancer Study Group Wilms' Tumour Study observed a lower incidence of 1.4% among 355 children with WT.[11,12] The incidence of 4.8%, in the present study, was comparable to incidence reported in the literature. The diagnosis is essentially clinical, the thrombocytopenia and coagulopathy inherent in SOS, precluding a biopsy. In a study of liver biopsies obtained at the time of nephrectomy in 91 patients, evidence of SOS was present in 41 (45.1%).[13] Merely 12 patients (13.2%) satisfied the clinical criteria for SOS, suggesting that clinical SOS may represent only the tip of the iceberg.[13]
Proposed risk factors for Act-D induced SOS include younger age, right-sided tumors and radiotherapy.[5,14,15] These were not evident in our study, reiterating that any child exposed to Act-D is at potential risk of developing SOS. Although clinically indistinguishable from post-HSCT SOS which carries a high risk of mortality, Act-D induced SOS is predominantly reversible with timely diagnosis and robust management.[3,14] Possible factors which contribute to the higher mortality and irreversibility in post-HSCT SOS include: pre-existent endothelial damage secondary to hematological malignancies such as leukemia/lymphoma, direct as well as indirect injury to the endothelium by chemotherapeutic agents and radiation administered as conditioning regimen, and depletion of hepatic antioxidant glutathione reserves by alkylating agents such as busulfan.[16] Further, it has been observed that endothelial progenitor cells (markers of the endogenous regenerative potential of the endothelium) remain decreased for up to 2 years following allogenic HSCT, whereas they increase following exposure to chemotherapeutic agents such as cyclophosphamide.[16] All children receiving Act-D as part of chemotherapy require sentient monitoring for symptoms/signs of SOS. Most major series have employed the modified McDonald's criteria to diagnose Act-D-induced SOS.[4,9,11] The 3 criteria of jaundice, tender hepatomegaly, and/or weight gain/ascites were successfully applied to diagnose all the patients in the present study on a completely clinical basis. The clinical diagnosis is strongly supported by the presence of thrombocytopenia, conjugated hyperbilirubinemia and highly elevated serum transaminases on investigations.[14] Act-D induced SOS has also been referred to as hepatopathy-thrombocytopenia syndrome.[12,14,17] Thrombocytopenia in SOS is usually isolated, as compared to chemotherapy-induced myelosuppression which causes concomitant neutropenia.[14,18] All our patients had significant thrombocytopenia <50 href="https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5759062/#ref18" rid="ref18" class=" bibr popnode tag_hotlink tag_tooltip" id="__tag_659252948" role="button" aria-expanded="false" aria-haspopup="true" xss=removed>18] Four patients each had conjugated hyperbilirubinemia and elevated serum transaminases in our study. Bilirubin levels were modestly elevated (mean = 4.2 mg/dL). A similar observation of “mediocre bilirubinemia” was observed by a Polish study.[5] In contrast, serum transaminases are elevated to more than 5 times upper limit of normal, with serum aspartate transaminase being higher than serum alanine transaminase.[4,5,14,18] Findings on abdominal ultra-sonogram described in SOS include thickened gall bladder wall, reversed/attenuated portal venous blood flow and ascites.[4] Ascites alone was demonstrable in our patients. It is possible that presence of ascites obscured other findings in our patients.
The cornerstone of management in Act-D-induced SOS is supportive care. Fluid restriction, intensive monitoring of fluid balance (input/output), appropriate use of diuretics, judicious transfusion of blood products, albumin infusion, and administration of broad spectrum antibiotics are important components of supportive care.[18] Vasoactive drug infusion, ventilation, renal replacement therapy may be required with the onset of multi-organ dysfunction. Besides supportive care, specific interventions are highly variable and are extrapolated from the management of post-HSCT SOS and fulminant liver failure due to other causes. Steroids, recombinant tissue plasminogen activator, antithrombin, and inhaled nitric oxide have been used with varying results in post-HSCT SOS as well as Act-D-induced SOS.[4,5,18,19] N-acetylcysteine has shown improvement in survival in fulminant hepatic failure secondary to other causes.[20] There are reports of the use of NAC in Act-D-induced SOS.[21] We used it in two patients who had signs of encephalopathy, both of whom recovered completely. Defibrotide has been shown to improve complete response rates as well as survival in severe SOS following HSCT and Act induced SOS with relatively lesser toxicity when compared to other drugs.[4,17,22] It is currently approved by the FDA for use in post-HSCT SOS in both children and adults, at a dose of 6.25 mg/kg every 6 h given as a 2-hour intravenous infusion for a minimum period of 21 days.[23] We were unable to use the drug due to limited availability and exorbitant cost. We have managed our patients without defibrotide. Although it was given in 2 children, the duration was minimal. Mortality rate secondary to Act-D ranges from 0 to 10% in large series.[18] One among the 5 children in this analysis expired.
Act-D can be safely re-exposed after complete recovery from SOS, starting at 50% dose and gradually escalating to full dose if tolerated well.[4,5,6] However, the decision is conscientious, and after severe, life-threatening episodes, certain physicians may prefer to avoid Act-D re-exposure.[18] In the study, two were safely re-exposed to Act-D while we consciously replaced further doses of Act-D with doxorubicin in another patient.
Conclusions
Act-D-induced SOS is a life-threatening complication during treatment for WT, which otherwise has an excellent prognosis. Diagnosis is clinical and supported by simple investigations such as blood count, liver function tests, and abdominal ultrasound. Supportive care can salvage the majority of patients, as it is reversible in most cases. Act-D can be re-exposed after complete recovery under close monitoring.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- PDQ Pediatric Treatment Editorial Board. Wilms Tumor and Other Childhood Kidney Tumors Treatment (PDQ ®): Health Professional Version. In: PDQ Cancer Information Summaries. Bethesda (MD): National Cancer Institute (US); 2002. Available from: http://www.ncbi.nlm.nih.gov/books/NBK65842/. [Last accessed on 2016 Aug 11].
- Szychot E, Apps J, Pritchard-Jones K. Wilms' tumor: Biology, diagnosis and treatment. Transl Pediatr 2014;3:12-24.
- Hopps SA, Borders EB, Hagemann TM. Prophylaxis and treatment recommendations for sinusoidal obstruction syndrome in adult and pediatric patients undergoing hematopoietic stem cell transplant: A review of the literature. J Oncol Pharm Pract 2016;22:496-510.
- Cesaro S, Spiller M, Sartori MT, Alaggio R, Peruzzo M, Saggiorato G, et al. Veno-occlusive disease in pediatric patients affected by Wilms tumor. Pediatr Blood Cancer 2011;57:258-61.
- Czauderna P, Katski K, Kowalczyk J, Kurylak A, Lopatka B, Skotnicka-Klonowicz G, et al. Venoocclusive liver disease (VOD) as acomplication of Wilms' tumour management in the series of consecutive 206 patients. Eur J Pediatr Surg 2000;10:300-3.
- Sulis ML, Bessmertny O, Granowetter L, Weiner M, Kelly KM. Veno-occlusive disease in pediatric patients receiving actinomycin D and vincristine only for the treatment of rhabdomyosarcoma. J Pediatr Hematol Oncol 2004;26:843-6.
- Cecen E, Uysal KM, Ozguven A, Gunes D, Irken G, Olgun N. Veno-occlusive disease in a child with rhabdomyosarcoma after conventional chemotherapy: Report of a case and review of the literature. Pediatr Hematol Oncol 2007;24:615-21.
- Kotecha RS, Buckland A, Phillips MB, Cole CH, Gottardo NG. Hepatic sinusoidal obstruction syndrome during chemotherapy for childhood medulloblastoma: Report of a case and review of the literature. J Pediatr Hematol Oncol 2014;36:76-80.
- McDonald GB, Hinds MS, Fisher LD, Schoch HG, Wolford JL, Banaji M, et al. Veno-occlusive disease of the liver and multiorgan failure after bone marrow transplantation: A cohort study of 355 patients. Ann Intern Med 1993;118:255-67.
- Pritchard-Jones K, Bergeron C, de Camargo B, van den Heuvel-Eibrink MM, Acha T, Godzinski J, et al. Omission of doxorubicin from the treatment of stage II-III, intermediate-risk Wilms' tumour (SIOP WT 2001): An open-label, non-inferiority, randomised controlled trial. Lancet 2015;386:1156-64.
- Ludwig R, Weirich A, Abel U, Hofmann W, Graf N, Tournade MF. Hepatotoxicity in patients treated according to the nephroblastoma trial and study SIOP-9/GPOH. Med Pediatr Oncol 1999;33:462-9.
- Raine J, Bowman A, Wallendszus K, Pritchard J. Hepatopathy-thrombocytopenia syndrome – Acomplication of dactinomycin therapy for Wilms' tumor: A report from the United Kingdom Childrens Cancer Study Group. J Clin Oncol 1991;9:268-73.
- Jagt CT, Zuckermann M, Ten Kate F, Taminiau JA, Dijkgraaf MG, Heij H, et al. Veno-occlusive disease as acomplication of preoperative chemotherapy for Wilms tumor: A clinico-pathological analysis. Pediatr Blood Cancer 2009;53:1211-5.
- Farruggia P, Macaluso A, Tropia S, Di Marco F, Russo D, Grigoli A, et al. Hepatopathy-thrombocytopenia syndrome (HTS) after actinomycin-D therapy: Report of three cases and review of the literature. Pediatr Hematol Oncol 2011;28:237-43.
- Tornesello A, Piciacchia D, Mastrangelo S, Lasorella A, Mastrangelo R. Veno-occlusive disease of the liver in right-sided Wilms' tumours. Eur J Cancer 1998;34:1220-3.
- Vion AC, Rautou PE, Durand F, Boulanger CM, Valla DC. Interplay of inflammation and endothelial dysfunction in bone marrow transplantation: Focus on hepatic veno-occlusive disease. Semin Thromb Hemost 2015;41:629-43.
- Martín-Lázaro JF, Palanca D, Garcia-Iñiguez JP, Madurga P, Carboné A. Hepatopathy-thrombocytopenia syndrome after actinomycin-D therapy: Treatment with defibrotide. Pediatr Hematol Oncol 2013;30:25-7.
- D'Antiga L, Baker A, Pritchard J, Pryor D, Mieli-Vergani G. Veno-occlusive disease with multi-organ involvement following actinomycin-D. Eur J Cancer 2001;37:1141-8.
- Mertens R, Brost H, Granzen B, Nowak-Göttl U. Antithrombin treatment of severe hepatic veno-occlusive disease in children with cancer. Eur J Pediatr 1999;158 Suppl 3:S154-8.
- Lee WM, Hynan LS, Rossaro L, Fontana RJ, Stravitz RT, Larson AM, et al. Intravenous N-acetylcysteine improves transplant-free survival in early stage non-acetaminophen acute liver failure. Gastroenterology 2009;137:856-64, 864.e1.
- Lee AC, Goh PY. Dactinomycin-induced hepatic sinusoidal obstruction syndrome responding to treatment with N-acetylcysteine. J Cancer 2011;2:527-31.
- Choi A, Kang YK, Lim S, Kim DH, Lim JS, Lee JA. Severe hepatic sinusoidal obstruction syndrome in a child receiving vincristine, actinomycin-D, and cyclophosphamide for rhabdomyosarcoma: Successful treatment with defibrotide. Cancer Res Treat 2016;48:1443-7.
References
- PDQ Pediatric Treatment Editorial Board. Wilms Tumor and Other Childhood Kidney Tumors Treatment (PDQ ®): Health Professional Version. In: PDQ Cancer Information Summaries. Bethesda (MD): National Cancer Institute (US); 2002. Available from: http://www.ncbi.nlm.nih.gov/books/NBK65842/. [Last accessed on 2016 Aug 11].
- Szychot E, Apps J, Pritchard-Jones K. Wilms' tumor: Biology, diagnosis and treatment. Transl Pediatr 2014;3:12-24.
- Hopps SA, Borders EB, Hagemann TM. Prophylaxis and treatment recommendations for sinusoidal obstruction syndrome in adult and pediatric patients undergoing hematopoietic stem cell transplant: A review of the literature. J Oncol Pharm Pract 2016;22:496-510.
- Cesaro S, Spiller M, Sartori MT, Alaggio R, Peruzzo M, Saggiorato G, et al. Veno-occlusive disease in pediatric patients affected by Wilms tumor. Pediatr Blood Cancer 2011;57:258-61.
- Czauderna P, Katski K, Kowalczyk J, Kurylak A, Lopatka B, Skotnicka-Klonowicz G, et al. Venoocclusive liver disease (VOD) as acomplication of Wilms' tumour management in the series of consecutive 206 patients. Eur J Pediatr Surg 2000;10:300-3.
- Sulis ML, Bessmertny O, Granowetter L, Weiner M, Kelly KM. Veno-occlusive disease in pediatric patients receiving actinomycin D and vincristine only for the treatment of rhabdomyosarcoma. J Pediatr Hematol Oncol 2004;26:843-6.
- Cecen E, Uysal KM, Ozguven A, Gunes D, Irken G, Olgun N. Veno-occlusive disease in a child with rhabdomyosarcoma after conventional chemotherapy: Report of a case and review of the literature. Pediatr Hematol Oncol 2007;24:615-21.
- Kotecha RS, Buckland A, Phillips MB, Cole CH, Gottardo NG. Hepatic sinusoidal obstruction syndrome during chemotherapy for childhood medulloblastoma: Report of a case and review of the literature. J Pediatr Hematol Oncol 2014;36:76-80.
- McDonald GB, Hinds MS, Fisher LD, Schoch HG, Wolford JL, Banaji M, et al. Veno-occlusive disease of the liver and multiorgan failure after bone marrow transplantation: A cohort study of 355 patients. Ann Intern Med 1993;118:255-67.
- Pritchard-Jones K, Bergeron C, de Camargo B, van den Heuvel-Eibrink MM, Acha T, Godzinski J, et al. Omission of doxorubicin from the treatment of stage II-III, intermediate-risk Wilms' tumour (SIOP WT 2001): An open-label, non-inferiority, randomised controlled trial. Lancet 2015;386:1156-64.
- Ludwig R, Weirich A, Abel U, Hofmann W, Graf N, Tournade MF. Hepatotoxicity in patients treated according to the nephroblastoma trial and study SIOP-9/GPOH. Med Pediatr Oncol 1999;33:462-9.
- Raine J, Bowman A, Wallendszus K, Pritchard J. Hepatopathy-thrombocytopenia syndrome – Acomplication of dactinomycin therapy for Wilms' tumor: A report from the United Kingdom Childrens Cancer Study Group. J Clin Oncol 1991;9:268-73.
- Jagt CT, Zuckermann M, Ten Kate F, Taminiau JA, Dijkgraaf MG, Heij H, et al. Veno-occlusive disease as acomplication of preoperative chemotherapy for Wilms tumor: A clinico-pathological analysis. Pediatr Blood Cancer 2009;53:1211-5.
- Farruggia P, Macaluso A, Tropia S, Di Marco F, Russo D, Grigoli A, et al. Hepatopathy-thrombocytopenia syndrome (HTS) after actinomycin-D therapy: Report of three cases and review of the literature. Pediatr Hematol Oncol 2011;28:237-43.
- Tornesello A, Piciacchia D, Mastrangelo S, Lasorella A, Mastrangelo R. Veno-occlusive disease of the liver in right-sided Wilms' tumours. Eur J Cancer 1998;34:1220-3.
- Vion AC, Rautou PE, Durand F, Boulanger CM, Valla DC. Interplay of inflammation and endothelial dysfunction in bone marrow transplantation: Focus on hepatic veno-occlusive disease. Semin Thromb Hemost 2015;41:629-43.
- Martín-Lázaro JF, Palanca D, Garcia-Iñiguez JP, Madurga P, Carboné A. Hepatopathy-thrombocytopenia syndrome after actinomycin-D therapy: Treatment with defibrotide. Pediatr Hematol Oncol 2013;30:25-7.
- D'Antiga L, Baker A, Pritchard J, Pryor D, Mieli-Vergani G. Veno-occlusive disease with multi-organ involvement following actinomycin-D. Eur J Cancer 2001;37:1141-8.
- Mertens R, Brost H, Granzen B, Nowak-Göttl U. Antithrombin treatment of severe hepatic veno-occlusive disease in children with cancer. Eur J Pediatr 1999;158 Suppl 3:S154-8.
- Lee WM, Hynan LS, Rossaro L, Fontana RJ, Stravitz RT, Larson AM, et al. Intravenous N-acetylcysteine improves transplant-free survival in early stage non-acetaminophen acute liver failure. Gastroenterology 2009;137:856-64, 864.e1.
- Lee AC, Goh PY. Dactinomycin-induced hepatic sinusoidal obstruction syndrome responding to treatment with N-acetylcysteine. J Cancer 2011;2:527-31.
- Choi A, Kang YK, Lim S, Kim DH, Lim JS, Lee JA. Severe hepatic sinusoidal obstruction syndrome in a child receiving vincristine, actinomycin-D, and cyclophosphamide for rhabdomyosarcoma: Successful treatment with defibrotide. Cancer Res Treat 2016;48:1443-7.


 PDF
PDF  Views
Views  Share
Share

