Study of histopathological features and proliferation markers in cases of Wilms? tumor
CC BY-NC-ND 4.0 · Indian J Med Paediatr Oncol 2012; 33(02): 102-106
DOI: DOI: 10.4103/0971-5851.99744
Abstract
Context: The spectrum of pediatric renal tumors is different from adult renal tumors, and Wilms′ tumor (WT) forms the majority. The histological type and clinicopathological staging are the two important prognostic parameters. The role of newer prognostic factors is not clear. Aims: This study was performed to analyze the histopathological spectrum of pediatric renal tumors and to study the expression of proliferation markers (Ki-67 and p53) in WT and correlate its expression in epithelial and blastema components in different stages. Materials and Methods: Twenty-seven cases of pediatric renal tumors were collected over 2 years. Hematoxylin-eosin staining was used for diagnosis. Immunostaining was performed for Ki-67 and p53. Ki-67 proliferation index (PI) and p53 expression were determined in each case and for the epithelial and blastema components separately. Statistical Analysis and Results: We had 20 cases of WT (74.1%), three cases of mesoblastic nephroma (11.1%), three cases of clear cell sarcoma (11.1%) and one case of rhabdoid tumor (3.7%). It was observed that the PI of the epithelial component (57.2%) was significantly higher than that of blastema (39.53%) in all stages. The PI in Stage II is significantly higher than that in Stage I. Statistical analysis could not be performed in Stages III and IV due to the small number of cases. p53 expression did not show any significant difference in the epithelial and blastema components. There was also no significant difference between the stages. Conclusion: In this study, we found the differences between PI of different tissue components of WT, with the epithelial component having a higher PI, which correlated with the stage of advancement of the disease.
Publication History
Article published online:
13 April 2022
© 2012. Indian Society of Medical and Paediatric Oncology. This is an open access article published by Thieme under the terms of the Creative Commons Attribution-NonDerivative-NonCommercial-License, permitting copying and reproduction so long as the original work is given appropriate credit. Contents may not be used for commercial purposes, or adapted, remixed, transformed or built upon. (https://creativecommons.org/licenses/by-nc-nd/4.0/.)
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India
Abstract
Context:
The spectrum of pediatric renal tumors is different from adult renal tumors, and Wilms’ tumor (WT) forms the majority. The histological type and clinicopathological staging are the two important prognostic parameters. The role of newer prognostic factors is not clear.
Aims:
This study was performed to analyze the histopathological spectrum of pediatric renal tumors and to study the expression of proliferation markers (Ki-67 and p53) in WT and correlate its expression in epithelial and blastema components in different stages.
Materials and Methods:
Twenty-seven cases of pediatric renal tumors were collected over 2 years. Hematoxylin-eosin staining was used for diagnosis. Immunostaining was performed for Ki-67 and p53. Ki-67 proliferation index (PI) and p53 expression were determined in each case and for the epithelial and blastema components separately.
Statistical Analysis and Results:
We had 20 cases of WT (74.1%), three cases of mesoblastic nephroma (11.1%), three cases of clear cell sarcoma (11.1%) and one case of rhabdoid tumor (3.7%). It was observed that the PI of the epithelial component (57.2%) was significantly higher than that of blastema (39.53%) in all stages. The PI in Stage II is significantly higher than that in Stage I. Statistical analysis could not be performed in Stages III and IV due to the small number of cases. p53 expression did not show any significant difference in the epithelial and blastema components. There was also no significant difference between the stages.
Conclusion:
In this study, we found the differences between PI of different tissue components of WT, with the epithelial component having a higher PI, which correlated with the stage of advancement of the disease.
INTRODUCTION
There are different types of pediatric renal tumors, like Wilms’ tumor (WT), mesoblastic nephroma, multicystic nephroma, clear cell sarcoma, rhabdoid tumor, nephroblastomatosis, intrarenal neuroblastoma, etc. Among these, WT or nephroblastoma is the most common one (85%).[1] It is seen primarily in infants, with 50% of the cases occurring below the age of 3 years.[2] There is no sex predilection. WT is usually sporadic, although 1-2% of the patients have a positive family history. Grossly, most cases of WT are large, solitary, gray-white and fleshy, well-circumscribed, rounded and soft in consistency. Cut-sections show predominantly solid pale areas with cystic change, necrosis and hemorrhage.[3] Microscopically, it comprises three major components, undifferentiated blastema, mesenchymal (stromal) tissue and epithelial tissue. When all the three components present together, then it is called a triphasic or classic WT, but it may be bi- or monophasic also.[4]
Additional morphologic features that can be encountered in WT include ciliated mucinous, endocrine cells of various types, renin-producing cells, neuroepithelium, neuroblast, rhabdomyoblast and mature ganglion cells, neuroglia, adipose tissue and cartilage, bone and hematopoietic cells.[3]
Among the different prognostic factors, stage and histologic type play important roles in assessing the prognosis and survival of patients with WT. Clinicopathological staging of WT is the single most important prognostic determinant. Capsular invasion, rupture at surgery, renal sinus involvement, renal vein invasion, tumor implants, lymph node metastasis and bilaterality are the main criteria used. Favorable and unfavorable histologic types have a great impact on prognosis. Other important prognostic factors are age, extensive tubular differentiation, skeletal muscle differentiation, mucin production, deoxyribonucleic acid (DNA) ploidy, p53 mutation, proliferation marker (MIB-I or Ki67), proliferating cell nuclear antigen (PCNA), p27 protein expression, vascular endothelial growth factor receptor (VEGFR), platelet derived growth factor receptor (PDGFR) and Type I insulin-like growth factor receptor expression have prognostic significance, but their roles are still not yet well established. This study was carried out to analyze the histopathological spectrum of pediatric renal tumors and to study the expression of proliferation marker (Ki-67) and p53 in WT and correlate its expression in epithelial and blastemal components in different stages.
MATERIALS AND METHODS
In this study, 27 cases were collected for a period of 2 years. Cases were collected and records were documented as per the following proforma: (a) identification: Name, age, sex, (b) Clinical findings, (c) Radiological findings and (d) Peroperative findings.
After receiving the specimen containing tumor in buffered formalin, macroscopical examination of the tumor was carried out. A graphic record of the site of each block was indicated on a photograph of the bisected specimen using a digital camera. Besides the adrenal glands, lymph nodes, renal vessels, renal sinus, capsule, pelvis and ureter wherever removed along with the kidney tumor, had been examined and sections were taken to make a pathological staging according to the National Wilms’ Tumor Study (NWTS-5) staging.
Hematoxylin-eosin stain was performed and used for tumor diagnosis, histologic type and staging. Further sections were taken on Poly-L-lysine-coated slides from tumor-containing blocks for immunohistochemistry for Ki-67 and p53 using a standard immunohistochemistry protocol.
We used the statistical data analysis software version 6.0. Tulsa Oklahoma: Statsoft Inc., 2001, for statistical analysis.
RESULTS AND ANALYSIS
We examined a total of 27 cases of pediatric renal tumors, of which 20 cases were WT (74.1%), three cases were mesoblastic nephroma (11.1%), three cases were clear cell sarcoma (11.1%) and one case was rhabdoid tumor (3.7%) [Figure 1a].
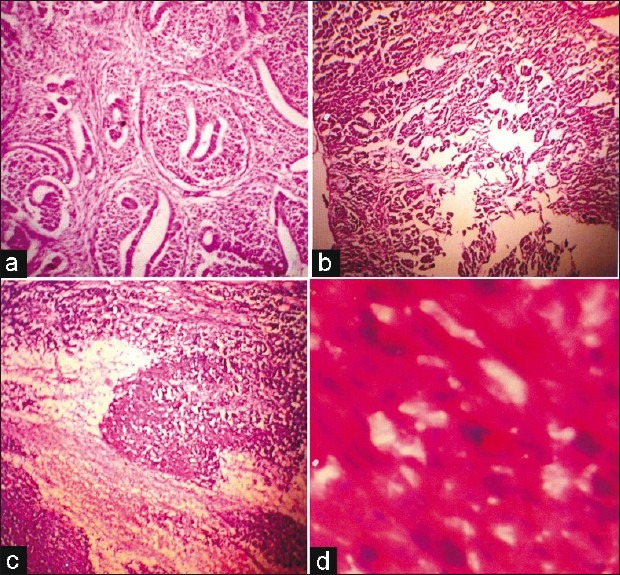
| Fig. 1 Photomicrograph of a case of triphasic Wilm's tumor (WT) [hematoxylin-eosin (H/E); ×LP] (a) A case of triphasic WT (H/E) (b) Low-power view of a case of epithelial predominant WT (H/E; ×LP) (c) High-power view of a case of blastema predominant WT (H/E; ×LP) (d) High-power view of unfavorable histology showing abnormal mitosis, nuclear enlargement and hyperchromasia (H/E; ×HP)
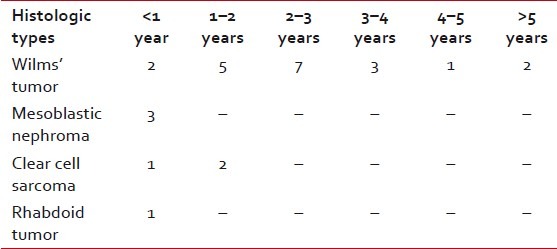
Regarding the age distribution of WT cases, it was seen that all the cases were in between 8 and 74 months, with a peak age incidence of 1-3 years of age [Table 1].
Table 1
Distribution of different histologic types of pediatric renal tumors according to age
On analyzing the sex distribution of WT, it was observed that 13 cases (65%) were males and seven cases (35%) were females, with a male: female ratio of 1.9:1.
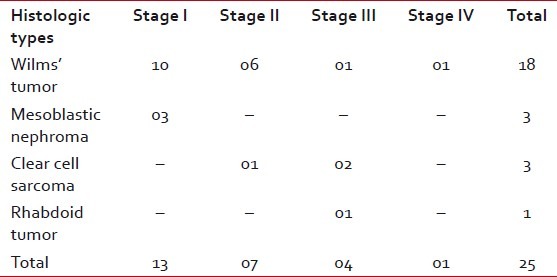
Of the 18 cases of WT, 10 cases presented in Stage I, six cases presented in Stage II, one case presented in Stage III and one case presented in Stage IV. Most cases of WT were in Stage I (55.55%) and in Stage II (33.33%) [Table 2]. In two cases, based on the computed tomography scan findings and fine needle aspiration cytology (FNAC) reports suggestive of WT, pre-operative chemotherapy was administered because of the large size. As these specimens showed extensive necrosis and fibrosis, NWTS-5 staging was not done and these two cases were excluded from the study.
Table 2
Distribution of pediatric renal tumors according to stages
Epithelial predominance [Figure 1b] and blastema predominance [Figure 1c] were equally distributed and did not show any correlation with the staging. Unfavorable histology was found in one [Figure 1d] out of 18 cases and, therefore, the percentage of unfavorable histology was 5.6%.
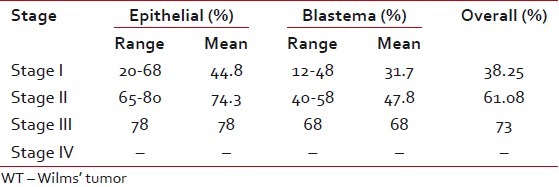
Ki-67 labeling index was determined for blastema and epithelial components separately. Of the 18 cases of WT, the range of Ki-67-positive cells in the epithelial component in Stage I was 20-68% (mean 44.8%) and in blastema this was 12-48% (mean 31.7%); in Stage II, the epithelial component was 65-80% (mean 74.3%) [Figure [Figure2a2a and andb]b] and in blastema it was 40-58% (mean 47.8%); in Stage III, in the epithelial component, this was 78% and in blastema it was 68% [Figure [Figure3a3a and andb].b]. Immunostaining of the single case of Stage IV could not be done as blocks were not available. Combined epithelial and blastemal Ki-67 expression in different stages were: Stage I-38.25%, Stage II-61.08% and Stage III-73%. Total proliferation index (PI) of the epithelial component was 57.2%, and in the blastema component it was 39.53% [Table 3].
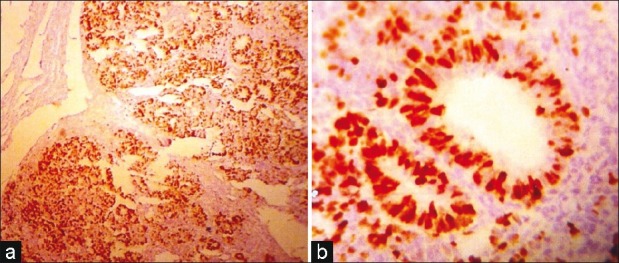
| Fig. 2 (a) Low-power view of very high Ki 67 labeling index in the epithelial component (IHC; ×LP) (b) High-power view of Case 2a (IHC; ×HP)
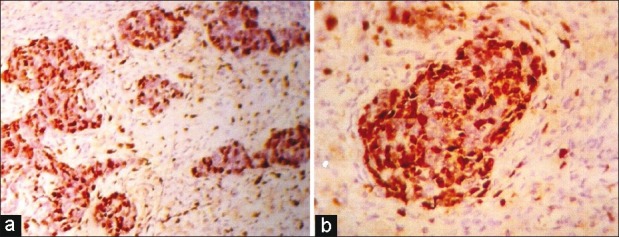
| Fig. 3 (a) Low-power view showing low Ki 67 labeling index in the stromal component than in the blastema component (IHC; ×LP) (b) High-power view showing Case 3a (IHC; ×HP)
Table 3
Range of Ki-67 proliferation index in WT and stage of advancement
On analyzing the data, it was found that the epithelial component had a significantly higher PI compared with the blastema component in Stage I (P=0.05) [Figure 4a], in Stage II (P=0.02) and in all stages together (P=0.002). Again, comparing the PI between the stages, it was found that Stage I showed a significantly lower PI compared with Stage II for the epithelial component (P=0.001) [Figure 4b], blastema component (P=0.03) and the epithelial and blastema components together (P=0.002).
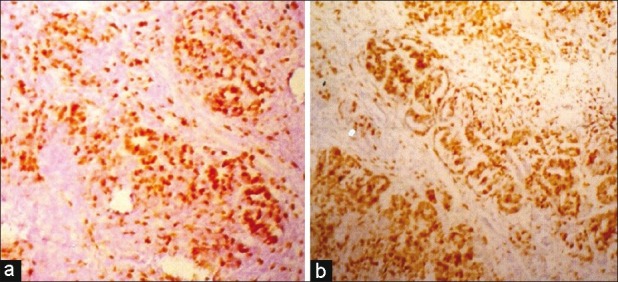
| Fig. 4 (a) Photomicrograph showing increased labeling index of Ki-67 in the epithelial component than that of blastema in Stage I (IHC; ×LP) (b) Photomicrograph showing Stage II Wilm's tumor with higher labeling index of Ki-67 than Stage I (IHC; ×LP)
Expression of p53 was performed in a semiquantitative manner as mild, moderate and marked. Only one case of Stage I, two cases of Stage II and one case of Stage III showed moderate expression of p53, and all the others showed a low level of expression.
p53 expression did not show any significant difference of expression between the epithelial and the blastema components. There was no significant difference between the stages. However, it was observed that the single case of Stage III had a higher intensity of staining than the other cases in the lower stages.
DISCUSSION
We have examined different histologic subtypes of cases of pediatric renal tumors in our study. Among a total of 27 cases of pediatric renal tumors, the incidence of WT was 74.1%, of mesoblastic nephroma was 11.1%, of clear cell sarcoma was 11.1% and of rhabdoid tumor was 3.7%. Our percentage of WT was less than that of the world literature, which is approximately 85%.[1] Because the world literature is based on Western data, this difference may partly be due to the small number of cases present in this study and regional variation due to unknown causes.
The peak age of WT cases in our study was 1-3 years. This peak age is less than the peak age of the world literature. However, our data matches with that of other Asian studies.[5]
Regarding sex distribution, in our study, there is a definite male predominance in WT (male:female=1.9:1), which is similar to the study by Mishra, et al. and other Asian studies, but the ratio is somewhat greater than that of American/European data.[5,6] This may be because of the social practices favoring the male child, who is more frequently brought to the hospital than the female child.
Analyzing the distribution of 18 WT cases in different stages according to clinicopathological staging, we had 10 cases (55.55%) in Stage I, six cases (33.33%) in Stage II and one case each (5.5%) in Stage III and Stage IV. Patient numbers in Stage I and II is greater than that in Stage III and IV, which correlates with other studies.[7,8]
Of the 18 cases, 17 cases (94.4%) showed a favorable histology and only one case (5.6%) had an unfavorable histology. This is similar to the percentage of unfavorable histology of 5% quoted in the Sternberg's Diagnostic Surgical Pathology.[9]
We have further analyzed the distribution of proliferation marker (Ki-67) and p53 in different stages of WT, with special reference to various histological components. In our study, it was observed that the PI of the epithelial component was significantly higher than that of blastema in all stages. Others, i.e. Khine, Ghanem and Juszkiewicz, have also made a similar observation; however, none of these studies give any plausible explanation for these observations. This is difficult to understand if the epithelial component is considered as a differentiation product from other components of the tumor. Nephroblastoma is a heterogenous tumor composed of epithelial, blastema and stromal components, and the epithelial component is expected to have the lowest proliferative activity. The dense blastema component, which is considered to be less-differentiated tissue from the epithelium, had a lower proliferation rate than the epithelial component.
Khine et al.[10] in a study similar to ours, with eight cases of pediatric nephroblastoma, noted that the PI of Ki-67 and PCNA of the epithelial component was higher than that of the other components.
Ghanem et al.[7] observed increased Ki-67 expression in the epithelial component over the blastema component. They showed that higher PI of Ki-67 was indicative of clinical progression and survival. Although we have not got survival data in our study, we found that the PI was higher in higher stages.
In a study by Juszkiewicz et al.,[11] the PI of neoplastic cells was determined in epithelial, blastema and stromal components of the tumors. In tumors after chemotherapy, a higher PI in epithelial component and a lower index in the blastema component was found.
Skotnicka-Klonowicz et al.[12] found that increased PCNA index in the epithelial component was associated with a higher stage of clinical advancement, as seen in our study.
It was also observed that PI in Stage II is significantly higher than Stage I for the individual component as well as overall. Statistical analysis could not be performed in other stages due to the small number of cases.
Sredni et al.[13] found that overall survival was higher for patients with p53 negative than with p53-positive cases, and they concluded that increased p53 expression in WT seems to be associated with advanced disease and relapse.
Huang et al.[14] showed that p53 accumulation in favorable histology WT is associated with angiogenesis and clinically aggressive disease.
Although this study performed on a small number of cases did not show any statistical significance between p53 expression and staging, higher intensity was noted in Stage II and III.
Literature on the significance of p53 in WT is more conflicting. In a study by Malkin et al.,[15] it was shown that p53 mutation was infrequently seen in WT. However, a later study by Lahoti et al.[16] had shown that p53 mutation correlated with recurrence and metastasis. Difference in staining pattern, staining protocol, antibody used and detection system used may be responsible for this discrepancy.
From this study, we found that in our set-up, WT forms 74.1% of all pediatric renal tumors, with a definite male predominance. Majority of the cases of WT presented in Stage I (55.55%) and Stage II (33.33%). Ki-67 PI was higher in the epithelial component in all stages. Ki-67 PI also increased with increased stage of advancement.
ACKNOWLEDGMENT
The authors are particularly thankful to the technical staff, Janababu, Ashimda and Ratnadi, who also actively helped in the work. The authors also owe special appreciation for their cooperation.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.

| Fig. 1 Photomicrograph of a case of triphasic Wilm's tumor (WT) [hematoxylin-eosin (H/E); ×LP] (a) A case of triphasic WT (H/E) (b) Low-power view of a case of epithelial predominant WT (H/E; ×LP) (c) High-power view of a case of blastema predominant WT (H/E; ×LP) (d) High-power view of unfavorable histology showing abnormal mitosis, nuclear enlargement and hyperchromasia (H/E; ×HP)

| Fig. 2 (a) Low-power view of very high Ki 67 labeling index in the epithelial component (IHC; ×LP) (b) High-power view of Case 2a (IHC; ×HP)

| Fig. 3 (a) Low-power view showing low Ki 67 labeling index in the stromal component than in the blastema component (IHC; ×LP) (b) High-power view showing Case 3a (IHC; ×HP)

| Fig. 4 (a) Photomicrograph showing increased labeling index of Ki-67 in the epithelial component than that of blastema in Stage I (IHC; ×LP) (b) Photomicrograph showing Stage II Wilm's tumor with higher labeling index of Ki-67 than Stage I (IHC; ×LP)


 PDF
PDF  Views
Views  Share
Share

