Subacute quadriplegic myelopathy following intrathecal methotrexate
CC BY-NC-ND 4.0 · Indian J Med Paediatr Oncol 2016; 37(03): 207-208
DOI: DOI: 10.4103/0971-5851.190361
Publication History
Article published online:
12 July 2021
© 2016. Indian Society of Medical and Paediatric Oncology. This is an open access article published by Thieme under the terms of the Creative Commons Attribution-NonDerivative-NonCommercial-License, permitting copying and reproduction so long as the original work is given appropriate credit. Contents may not be used for commercial purposes, or adapted, remixed, transformed or built upon. (https://creativecommons.org/licenses/by-nc-nd/4.0/.)
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India
Sir,
Chemotherapy is associated with a variety of central nervous system (CNS) complications. Intrathecal (IT) methotrexate (MTX) has been used extensively in neuro-oncology and myelopathy is well described in children and especially in adolescents.[1] Reports of myelopathy in adults are rare.[2] We report an Indian adult with IT-MTX induced quadriplegia. A 37-year-old man presented with complaints of headache for 1 week and intermittent left parotid swelling since 1 year. Magnetic resonance imaging (MRI) brain revealed a large lesion in the left temporal region with contiguous involvement of left buccal and masticator space, left petrous temporal bone erosions, and left basitemporal extradural soft tissue. Screening computed tomography (CT) showed permeative lysis of left temporal bone. He underwent left temporal craniotomy and excision of the brain lesion which revealed a high-grade non-Hodgkins's lymphoma diffuse large B-cell lymphoma. He underwent a positron emission tomography CT, bone marrow aspiration biopsy, parotid biopsy, and cerebrospinal fluid cytology which were all normal. A combined modality treatment with chemotherapy and radiation therapy with triple agent IT therapy was planned as he had significant local dural infiltration. He then underwent a chemoport insertion followed by a DeAngelis protocol chemotherapy (rituximab, MTX, procarbazine, and vincristine; and triple IT therapy MTX, cytarabine, and hydrocortisone) for possible spinal metastasis and skull base infiltration. One week after his last cycle of IT, he started complaining of tingling and numbness of both lower limbs. He then developed difficulty in walking difficulty, unsteady gait, and a girdle sensation around lower chest bilaterally. On examination, he was conscious and oriented. He had impaired vibration and joint position sensations in both lower limbs with sluggish deep tendon reflexes and extensor planters. An emergency MRI spine and nerve conduction studies were normal. A diagnosis of probable MTX-induced myelopathy was made. Further chemotherapy was postponed. However, he underwent whole brain irradiation after informing him of the possibility of worsening of myelopathy. He was started on injection methylcobalamin, folic acid, and vitamins. However, over 2 weeks, his weakness worsened to Grade 0 power in the legs, Grade 4 in the upper limbs and his sensory level ascended to D2 level. At this point, another MRI of the neuraxis with contrast showed complete resolution of previously documented extradural enhancing lesion with a significant reduction in previously documented contiguous lesion in the left preauricular soft tissue. The lower dorsal cord (D5–D11) showed a hyperintense signal in the posterior aspect without contrast enhancement [Figure 1]. At 1 month follow-up, he was still quadriplegic with Grade 4 power in the arms and Grade 0 in the legs and a sensory level at D2 level. Methylenetetrahydrofolate reductase (MTHFR) gene mutation was not performed due to financial constraints.
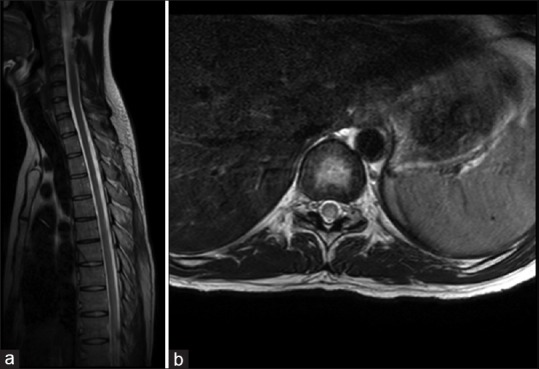
| Fig. 1 (a) Sagittal T2 magnetic resonance imaging of dorsal cord; subtle T2 hyperintensity in the dorsal cord. (b) Axial T2 magnetic resonance imaging of dorsal cord showing subtle posterior cord hyperintensity
Adverse effects of IT chemotherapy include arachnoiditis, lumbosacral radiculopathy, leukoencephalopathy, and myelopathy.[3] MRI findings include T2 hyperintensities in the central and posterior columns, cord swelling, or lateral column contrast enhancement. Vacuolar degeneration of the white matter is usually described in MTX myelopathy. Rarely extensive necrotic myelopathy has been noted.[4] Cytarabine myelopathy is rarer and has been described with extensive cord edema and peripheral cord enhancement.[5]
MTX is a competitive inhibitor of several enzymes involved in folate and methionine metabolism including dihydrofolate reductase, resulting in a marked increase of homocysteine within the CNS and to a deficiency of S-adenosylmethionine which is necessary for myelination, neuronal membrane stability and neurotransmission. Moreover, two MTHFR missense polymorphisms c.677C>T and c.1298A>C and a Tc2 c.776C>G polymorphism are associated with an increased risk of white matter changes in MTX therapy.[6] IT myelopathy has a variable prognosis although isolated case reports describe a good response to multiple folate metabolites.[7] It is important to recognize it early and discontinue IT therapy to avoid irreversible damage.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.


 PDF
PDF  Views
Views  Share
Share

