Successful outcome of mucormycosis in two children on induction therapy for acute lymphoblastic leukemia
CC BY-NC-ND 4.0 · Indian J Med Paediatr Oncol 2013; 34(04): 313-316
DOI: DOI: 10.4103/0971-5851.125254
Abstract
Zygomycetes are one of the less common causes of invasive fungal infections in patients with the hematological malignancies. We report two cases of acute lymphoblastic leukemia in the pediatric age group, complicated by disseminated cutaneous mucormycosis during induction chemotherapy. In one of our cases, cutaneous mucormycosis progressed to osteomyelitis of the proximal ulna while in the other it disseminated to lungs and distant cutaneous site. Aggressive treatment, which included intravenous administration of amphotericin B; radical surgical intervention in the form of serial wound debridement in the former and wedge pulmonary resection in the later, allowed successful salvage, and administration of planned anti-leukemia treatment in both of our cases. High index of suspicion, timely diagnosis, and appropriate treatment are key components of a successful outcome.
Publication History
Article published online:
19 July 2021
© 2013. Indian Society of Medical and Paediatric Oncology. This is an open access article published by Thieme under the terms of the Creative Commons Attribution-NonDerivative-NonCommercial-License, permitting copying and reproduction so long as the original work is given appropriate credit. Contents may not be used for commercial purposes, or adapted, remixed, transformed or built upon. (https://creativecommons.org/licenses/by-nc-nd/4.0/.)
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India
Abstract
Zygomycetes are one of the less common causes of invasive fungal infections in patients with the hematological malignancies. We report two cases of acute lymphoblastic leukemia in the pediatric age group, complicated by disseminated cutaneous mucormycosis during induction chemotherapy. In one of our cases, cutaneous mucormycosis progressed to osteomyelitis of the proximal ulna while in the other it disseminated to lungs and distant cutaneous site. Aggressive treatment, which included intravenous administration of amphotericin B; radical surgical intervention in the form of serial wound debridement in the former and wedge pulmonary resection in the later, allowed successful salvage, and administration of planned anti-leukemia treatment in both of our cases. High index of suspicion, timely diagnosis, and appropriate treatment are key components of a successful outcome.
INTRODUCTION
Mucormycosis is an invasive fungal infection caused by various members of the class Phycomycetes, especially Mucoraceae, subdivided into the genera Actinomucor, Rhizopus, Rhizomucor, and Mucor.[1] After aspergillosis, mucormycosis is the second most common mycosis caused by the filamentous fungi. Local trauma such as that caused by iv cannulation, use of adhesive tapes and arm boards in the pre-disposed individual (prolonged corticosteroids use as in an organ or bone marrow transplantation, prolonged, and profound neutropenia in malignant hematological disorders) is an important risk factor for cutaneous mucormycosis.[2,3] Although ubiquitous, Zygomycetes rarely cause disease in the absence of above mentioned risk factors. In hematological malignancies, especially in induction, this is associated with high mortality rate. Physician has to maintain a fine balance between antileukemic and antifungal therapy as both of these are equally important for long-term successful outcome. We report two such challenging cases of acute lymphoblastic leukemia (ALL) complicated by mucormycosis and successfully managed by timely and appropriate administration of antifungal agents and surgical intervention without compromising antileukemic therapy.
CASE REPORTS
Case 1
A 10-year-old male was on induction chemotherapy (BFMBerlin-Frankfurt-Münster-95 protocol) for moderate risk T cell ALL. On day 11 of induction, he was observed to have a scabbed wound (2 cm × 2 cm) on the lateral aspect of his left forearm. There was a history of trauma 4 days ago. As the wound was in close proximity to the central line, parenteral antibiotics were started immediately. Antibiotics were switched from cefoperazone-sulbactam to meropenem and amikacin after 48 hour of non-response. Although absolute neutrophil count (ANC) = 60/mm3, child remained afebrile, non-toxic, capillary refill was brisk and radial pulsations were well-felt. Repeated blood and wound swab cultures were sterile. The lesion progressed and conventional amphotericin-B (CAB) was started on D18 of induction, after taking a skin biopsy for culture and histology. Skin scrapping showed growth of non-septate hypha, branching at right angles, diagnostic of mucormycosis. CAB was continued for 12 weeks, along with radical serial debridement of the necrotic wound. Computed tomography (CT) chest was normal. Induction chemotherapy was continued with prednisolone, vincristine, and L-asparaginase, however, only first dose of daunorubicin could be administered. Skin grafting by rotation flap was carried out to cover the exposed ulna proximal to the styloid process [Figure 1]. He achieved complete remission (CR) and subsequent cycles of chemotherapy were administered as per the protocol. He completed his treatment and is off therapy for the past 7 months.
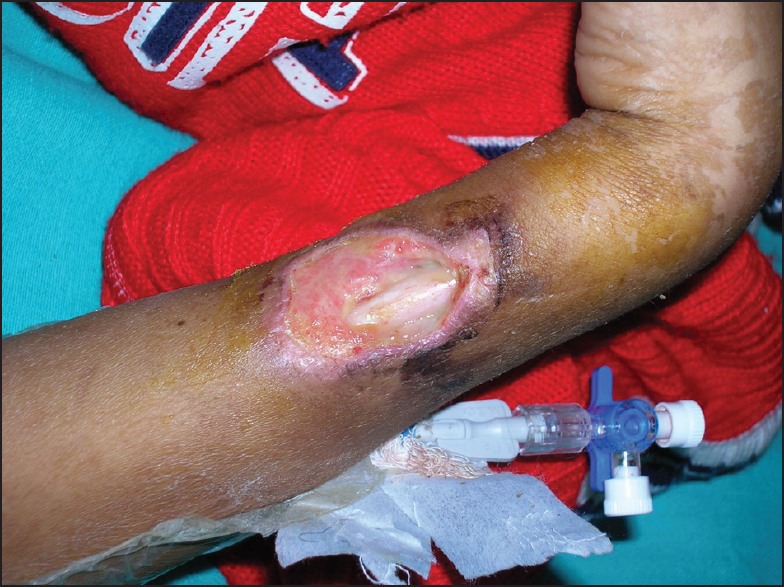
| Fig. 1 Cutaneous mucormycosis exposing the shaft of ulna (case 1)
Case 2
A 4-year-old male diagnosed with standard risk pre-cursor B ALL was hospitalized on day 4 of induction chemotherapy for fever and cellulitis on the dorsum of the right hand, at venous cannula site. As he was neutropenic (ANC = 100/mm3) broad spectrum antibiotics were administered to cover both gram positive and gram negative bacteria. The wound progressed to an abscess and pus culture grew antibiotic sensitive Pseudomonas aeruginosa. Despite the specific therapy (cefoperazone-sulbactam, amikacin), the patient developed a necrotic area at the tip of the little finger of the same hand, suggestive of ecthyma gangrenosum [Figure 2a]. He subsequently developed pain and swelling over the right shoulder and as he had a central line on the same arm (8 cm from the wound), the line was removed despite normal coagulation profile and Doppler study. Shoulder pain resolved within 24 h of removing the line. As child remained afebrile, non-toxic and hemodynamically stable, induction chemotherapy was continued with some modification (no daunorubicin after D8). On D19, he developed another black skin lesion on the lateral aspect of left leg [Figure 2b] and skin scrapping from the lesion grew Streptococci on initial culture. However, due to progression, biopsy from the leg lesion was done and liposomal amphotericin B (LAB) was started. Histopathological examination showed broad ribbon-like non-septate hypha branching at the right angle, suggestive of mucormycosis. The fungal culture showed fluffy, grey, cotton candy-like growth that was identified as Rhizomucor pussilus [Figure 2c]. CT scan of the chest revealed multiple, bilateral pulmonary lesions with right pleural effusion (at this time patient also had a cough and tachypnea). Successful control of mucormycosis was achieved by serial surgical debridement of both cutaneous lesions, wedge resection of two large lung lesions along with LAB and posaconazole (12 weeks). After completion of induction patient achieved CR and subsequent chemotherapy was administered as per protocol. He has completed his treatment and is off therapy for 4 months. The clinical characteristics of both cases are summarized in Table 1.
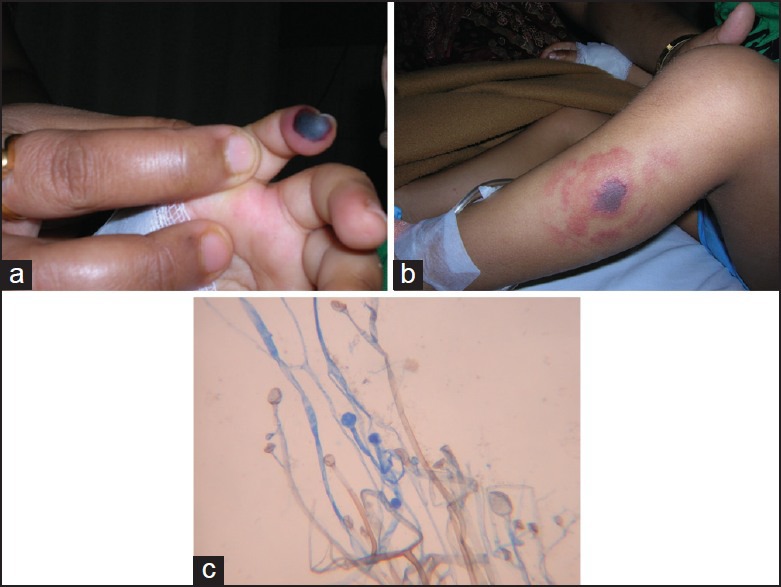
| Fig. 2 (a) Necrotic lesion on the distal end of 5th finger simulating ecthyma gangrenosum; (b) mucormycosis involving skin on the lateral aspect of left leg; (c) non-septate hypha branching at a right angle with un-branched sporangiophores and oval sporangium (case 2)
Table 1
Clinical characteristics of the two cases of ALL with mucormycosis

DISCUSSION
Zygomycosis is a frequently lethal fungal infection seen in association with neutropenia in post-transplant patients and those with the hematological malignancies.[4,5] Among patients with hematological malignancy, mucormycosis is associated more commonly with acute leukemia than with other conditions.[6] The most frequent causative agents are those belonging to the family Mucoraceae, genus Rhizopus, Rhizomucor, Absidia, Mucor, and Cunninghamella.[7] These are ubiquitous fungi inhabiting decomposing plants and animal matters. There can be various clinical presentations of mucormycosis including rhino-cerebral, pulmonary, cutaneous, gastrointestinal, central nervous system, and disseminated infections.
Cutaneous disease usually arises from primary inoculation at a site where dermal barrier has been breached, or occurs secondary to dissemination. Primary infection simulates acute inflammatory response leading to pus, abscess formation, tissue swelling, necrosis, often progressing to black eschars. These may slough off to leave ulcers and may mimic other cutaneous infections in immunocompromised patients, such as ecthyma gangrenosum, cutaneous aspergillosis or cutaneous fusariosis.[8] Our first case had primary cutaneous mucormycosis that was locally invasive while the second case had a cutaneous lesion to begin with that disseminated to involve the lungs and distant skin. Pre-disposing factors present in both cases were underlying leukemia, steroids, neutropenia, and immunosuppressive therapy.
Establishing a diagnosis is the central issue in the successful management of mucormycosis. Firstly, isolation of mucor from a potentially infected site may not be reliable in the absence of evidence of fungal invasion of tissue on histopathological examination. This is because of the ubiquitous nature of the organism and its ability to colonize normal persons. Secondly, the organisms may get killed on routine procedure of tissue grinding before fungal culture and so negative cultures may not rule out mucormycosis.[8] A negative initial culture report delayed the institution of appropriate therapy due to which our first case progressed to osteomyelitis. Thirdly, the isolation of bacteria from the lesions may dissuade the clinician from suspecting a concomitant fungal etiology; as in case 2, wherein a dissuading positive bacterial culture allowed a primary cutaneous lesion to disseminate. Fortunately though, both the cases had a successful outcome. There are reports of misdiagnosed mucormycosis infections in literature where the outcomes have been fatal.[9,10] Histological examination of biopsy specimen from the infected tissue shortens the time to establish the diagnosis. The biopsy specimen should demonstrate characteristic wide ribbon like aseptate hyphae branching at a right angle.[8] Nevertheless, isolating and identifying the causative fungus on culture is important for epidemiological and research purposes. In both of our cases, the histopathological examination of the affected tissue was consistent with these findings.
The factors that are important for a successful outcome of this infection are early diagnosis, reversal of any possible underlying pre-disposing factors such as neutropenia, aggressive surgical debridement of infected tissue, and appropriate antifungal therapy.[8] Deoxycholate amphotericin-B is the drug of choice for mucormycosis.[6,8] In vitro studies show that posaconazole is effective against zygomycetes especially Rhizopus species.[6,7,8] Voriconazole is not effective against mucormycosis. Kontoyiannis et al. have shown that the use of voriconazole is an independent risk factor for zygomycosis.[11] The timely administration of empirical amphotericin B on suspicion of fungal infection in both our cases along with debridement of necrotic tissue may be responsible for a successful outcome.
The mortality in patients with mucormycosis is as high as 50%. Mortality in patients with disseminated disease is as high as nearly 100%.[8] Jones et al.[12] document the only case in hand surgery where successful salvage of the Mucor infected forearm or hand in an immunocompromised patient could be achieved. In light of such data, the successful outcome in both our immunocompromised patients is highly encouraging.
In conclusion, therefore, the following points may be highlighted:
- It is often difficult to establish a diagnosis of mucormycosis with certainty, thereby leading to delay in administering appropriate antifungal therapy and a fatal outcome in turn. Hence a high degree of suspicion and low threshold for starting empirical antifungal are required.
- Amphotericin B and timely radical surgical debridement of all necrotic and infected tissue help in controlling the infection as well as salvaging the structural and functional features of the affected organ.
- Timely and appropriately aggressive interventions were responsible for a successful outcome despite dissemination, and highlighted the importance of judicious concomitant administration of antifungal and anti-leukemia treatment during induction therapy in ALL.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES

| Fig. 1 Cutaneous mucormycosis exposing the shaft of ulna (case 1)

| Fig. 2 (a) Necrotic lesion on the distal end of 5th finger simulating ecthyma gangrenosum; (b) mucormycosis involving skin on the lateral aspect of left leg; (c) non-septate hypha branching at a right angle with un-branched sporangiophores and oval sporangium (case 2)


 PDF
PDF  Views
Views  Share
Share

