Successful treatment of vincristine induced ptosis and polyneuropathy with pyridoxine and pyridostigmine in a child with acute lymphoblastic leukemia
CC BY-NC-ND 4.0 · Indian J Med Paediatr Oncol 2012; 33(03): 185-187
DOI: DOI: 10.4103/0971-5851.103152
Abstract
Vincristine is used in the treatment of solid tumors, lymphoma and leukemia in children. The dose-limiting toxicity is its neurotoxicity. We describe a 2-year-old girl with acute lymphoblastic leukemia who developed vincristine-induced polyneuropathy with bilateral ptosis and recovered on treatment with pyridoxine and pyridostigmine.
Publication History
Article published online:
02 August 2021
© 2012. Indian Society of Medical and Paediatric Oncology. This is an open access article published by Thieme under the terms of the Creative Commons Attribution-NonDerivative-NonCommercial-License, permitting copying and reproduction so long as the original work is given appropriate credit. Contents may not be used for commercial purposes, or adapted, remixed, transformed or built upon. (https://creativecommons.org/licenses/by-nc-nd/4.0/.)
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India
Abstract
Vincristine is used in the treatment of solid tumors, lymphoma and leukemia in children. The dose-limiting toxicity is its neurotoxicity. We describe a 2-year-old girl with acute lymphoblastic leukemia who developed vincristine-induced polyneuropathy with bilateral ptosis and recovered on treatment with pyridoxine and pyridostigmine.
INTRODUCTION
Vincristine is a vinca alkaloid used in combination with other agents in the treatment of pediatric malignancies. The dose-limiting neurotoxicity of vincristine is well recognised as it may lead to peripheral, autonomic and cranial polyneuropathy and rarely encephalopathy. Cranial nerve palsies involving oculomotor, trochlear and facial nerves are seen less frequently than peripheral neuropathy. Among ocular findings, ptosis and ophthalmoplegias are the most common manifestations.[1] We describe a 2-year-old girl with vincristine-induced polyneuropathy with bilateral ptosis and complete recovery of ptosis following treatment with pyridoxine and pyridostigmine. To the best of our knowledge, so far, less than five case reports are available in the literature on treatment of vincristine induced neuropathy.[2–5]
CASE REPORT
A 2-year-old girl with precursor B cell acute lymphoblastic leukemia (ALL) was started on treatment according to the MCP 841 protocol. She had grade 3 protein energy malnutrition at admission. She developed febrile neutropenia with sepsis during the third week of induction. She was treated with parenteral antibiotics and other supportive care. She had received four doses of vincristine (cumulative dose of 2.8 mg) and, on the 25th day of induction, she developed bilateral ptosis. She was afebrile at that time [Figure 1]. Neurological examination revealed bilateral ptosis and complete external opthalmoplegia with normal pupillary and corneal reflexes. She had hypotonia in both lower limbs with power of 2/5 with normal upper limb power, and there was no head lag. There was no ascending or descending pattern of weakness noticed. Bilateral knee and ankle jerks were not elicitable. There was no feature of autonomic neuropathy. Nerve conduction velocity showed motor axonal polyneuropathy with normal sensory conduction. Cerebrospinal fluid analysis showed normal protein, sugar and no cellular response. Her stool culture did not grow any organism. She was not on any drugs that could potentiate neurotoxicity of vincristine. Serum electrolytes and calcium levels were normal. Her polyneuropathy was static but ptosis progressively increased. A neuroprotective and neuroregenerative treatment attempt with pyridoxine (3 mg/kg Twice daily, per orally (BID, PO)) and pyridostigmine (150 mg/m2 BID, PO) was started. Bilateral ptosis, hypotonia and muscle weakness markedly improved after 14 days of pyridoxine and pyridostigmine treatment and ptosis completely resolved after 4 weeks. ([Figure 2]-recovery of ptosis after 2 weeks of treatment). Both the agents were given for 8 weeks and were well tolerated without any side-effects. There was no recurrence following cessation of pyridoxine and pyridostigmine. Because vincristine is an important drug in the treatment of ALL, after 2 weeks of complete recovery of ptosis, it was reinitiated in lower dose and later increased to the full dose without recurrence of neuropathy. She has received vincristine cumulative dose of 6.1 mg (10 mg/m2) till now and she is in maintenance phase of chemotherapy.
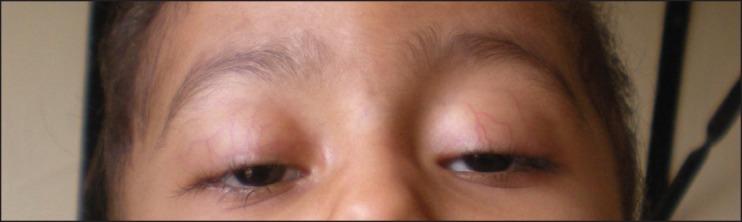
| Fig. 1 Figure showing bilateral ptosis
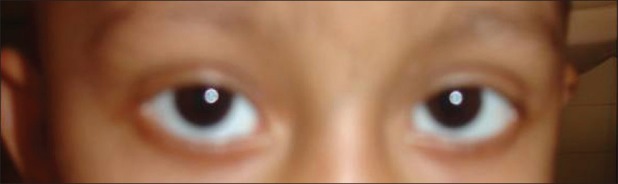
| Fig. 2 Complete recovery of ptosis after 2 weeks of treatment
DISCUSSION
Neurotoxicity is a known adverse effect of several chemotherapeutic agents like vincristine, cisplatin, oxaliplatin, docetaxel, paclitaxel and bortezomib. Of these, vincristine is commonly used in the treatment of pediatric cancers. Incidence of neurotoxicity is reported from 3 to 13% by few authors earlier, but the latest study from India reported a higher (50%) incidence.[6] This may partly be accounted for by the fact that the Indian patients who developed neurotoxicity were all severely malnourished, predisposing them to neurotoxicity.[7,8] Our patient also had severe malnutrition and, through the treatment, she further lost weight, which could have precipitated her to this adverse effect.
Axonal transport dysfunction is a major theory for the pathogenesis of a variety of toxic neuropathies, including vincristine-induced neurotoxicity.[9] The axon is more vulnerable than the pericaryon to the toxic effects of vincristine, specifically the distal axon as per another hypothesis.
Vincristine-induced neurotoxicity manifests as loss of deep tendon reflexes, neuritic pain, paresthesias and wrist and foot drop. Less frequently, cranial nerve palsies, transient cortical blindness, oculomotor nerve dysfunction, jaw pain, facial palsy, sensorineural hearing loss and laryngeal nerve paresis have been attributed to vincristine.[2] Symptoms usually appear 2–19 weeks after the commencement of vincristine. Vincristine neurotoxicity may be aggravated by higher dosage (>30–50 mg), hypersensitivity to the drug, pre-existing liver dysfunction or a hereditary neuropathy, and concomitantly use of other drugs such as allopurinol, erythromycin, isoniazid, mitomycin C, phenytoin and itraconazole.[10] In our patient, we did not use high-dose vincristine and the other concomitant accelerating drugs. Development of ptosis and ophthalmoplegia in relation to the therapeutic schedule with vincristine, resolution of symptoms after pyridoxine and pyridostigmine therapy, nerve conduction study showing acute motor axonal neuropathy and normal cerebrospinal fluid findings favor the diagnosis of vincristine-induced neuropathy.
Because the pathophysiology of vincristine neuropathy is not fully understood, preventive and therapeutic approaches are still experimental. A few case reports of full recovery of vincristine-associated bilateral ptosis (cranial polyneuropathy) after treatment with pyridoxine and pyridostigmine[2–5,11] are available. In those cases of vincristine-induced neuropathy treated with pyridoxine and pyridostigmine, there were isolated cranial neuropathies without peripheral neuropathy and, in our case, there were both cranial and peripheral neuropathies. A report by Ozgur Duman et al. described a patient with vincristine neurotoxicity with ptosis and facial nerve palsy and treated with pyridoxine alone, which showed complete recovery.[5]
Most of the neuropathic symptoms of vincristine toxicity are reversible within months or years after adjusting the dosage or achieving elimination of vincristine, although some are permanent. Nevertheless, the cranial neuropathic signs improved significantly in 5–7 days and completely resolved in 2–3 weeks in cases treated with pyridoxine and pyridostigmine. We used the same treatment regimen in our patient, and this was very well tolerated with no documented side-effects.
CONCLUSION
Children receiving vincristine need to be monitored closely for dose-limiting complications leading to the development of usually reversible neurotoxicity. Combined treatment with pyridoxine and pyridostigmine may hasten the clinical improvement even in severe cases.
ACKNOWLEDGMENT
We acknowledge the cooperation from the parents of the child during the treatment and for the giving consent to publish this case report.
Footnotes
Source of Support: Nil,
Conflict of Interest: None declared
REFERENCES

| Fig. 1 Figure showing bilateral ptosis

| Fig. 2 Complete recovery of ptosis after 2 weeks of treatment


 PDF
PDF  Views
Views  Share
Share

