Surveillance and expected outcome of acute lymphoblastic leukemia in children and adolescents: An experience from Eastern India
CC BY-NC-ND 4.0 · Indian J Med Paediatr Oncol 2013; 34(04): 280-282
DOI: DOI: 10.4103/0971-5851.125245
Abstract
Objective: Research in Eastern India especially among children and adolescents for acute lymphoblastic leukemia (ALL) have not been well documented until recently when it was conducted at a cancer institute of tertiary care with primary objectives of examining and correlating different cell surface markers involved with respect to disease surveillance thereby highlighting it as a strong prognostic marker for future diagnosis and treatment. Materials and Methods: A total of 500 consecutively selected ALL patients were diagnosed and treated according to National Cancer Institute protocol (MCP 841) for a period of 24-88 months during this hospital-based study. Results: Of the total, 50.4% had a higher incidence of T-ALL and 47.6% had pro-B, B-cell precursor ALL. Disease free survival and event free survival were remarkably higher in B-ALL adolescent patients as compared to T-ALL, who had significantly lower overall survival ratio. Prevalence of T-ALL was also observed in relapse cases for adolescent patients. Conclusions: We conclude that there is an increased prevalence of T-ALL among adolescents in Eastern India. Immunophenotypic analysis might help in proper evaluation and prediction of treatment outcomes with an increased thrust on studying age-specific incident rates enabling well planned future treatments for improved and better outcome.
Keywords
Acute lymphoblastic leukemia - B-acute lymphoblastic leukemia - immunophenotype - prognostic marker - T-acute lymphoblastic leukemiaPublication History
Article published online:
19 July 2021
© 2013. Indian Society of Medical and Paediatric Oncology. This is an open access article published by Thieme under the terms of the Creative Commons Attribution-NonDerivative-NonCommercial-License, permitting copying and reproduction so long as the original work is given appropriate credit. Contents may not be used for commercial purposes, or adapted, remixed, transformed or built upon. (https://creativecommons.org/licenses/by-nc-nd/4.0/.)
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India
Abstract
Objective:
Research in Eastern India especially among children and adolescents for acute lymphoblastic leukemia (ALL) have not been well documented until recently when it was conducted at a cancer institute of tertiary care with primary objectives of examining and correlating different cell surface markers involved with respect to disease surveillance thereby highlighting it as a strong prognostic marker for future diagnosis and treatment.
Materials and Methods:
A total of 500 consecutively selected ALL patients were diagnosed and treated according to National Cancer Institute protocol (MCP 841) for a period of 24-88 months during this hospital-based study.
Results:
Of the total, 50.4% had a higher incidence of T-ALL and 47.6% had pro-B, B-cell precursor ALL. Disease free survival and event free survival were remarkably higher in B-ALL adolescent patients as compared to T-ALL, who had significantly lower overall survival ratio. Prevalence of T-ALL was also observed in relapse cases for adolescent patients.
Conclusions:
We conclude that there is an increased prevalence of T-ALL among adolescents in Eastern India. Immunophenotypic analysis might help in proper evaluation and prediction of treatment outcomes with an increased thrust on studying age-specific incident rates enabling well planned future treatments for improved and better outcome.
INTRODUCTION
Acute lymphoblastic leukemia (ALL) is the most frequently diagnosed malignancy in children, representing nearly one third of all pediatric cancers.[1] ALL involves a specific subtype of lymphocyte, the T- or B-cell.[2] However, being a lymphoid neoplasm, variations exist in the prevalence of subtypes of ALL, in relation to geographic, environmental, socio-economic, ethnic and racial factors.[3] Although various clinical and laboratory prognostic factors like age, sex, hemoglobin level, white blood count (WBC) count and response to initial treatment etc., are used to predict whether a patient will remain in remission or not, immunophenotyping involving different cell surface markers may be considered as an important prognostic factor and also predictor of the outcome.
Variations of ALL and its subtypes, especially among children and adolescents in Eastern India, have not been well documented till date. Thus, the objective of the present study is to examine and correlate variety of cell surface markers in leukemia patients of children and adolescents with respect to disease surveillance and outcome.
MATERIALS AND METHODS
Patients
ALL patients presenting to Netaji Subhas Chandra Bose Cancer Research Institute (NCRI), a cancer institute of tertiary care, between March 31, 2004 and July 31, 2011 were included in the present study. Peripheral blood and/or bone marrow samples were obtained from 500 ALL patients, within the age range of 2-18 years (median age 10 years), prior to treatment. The study group comprised of individuals from both sexes (male and female ratio 3:2) including 321 pediatric (2-12 years) and 179 adolescents (13-18 years). No individuals below 2 years and/or above 18 years were included in this study. Patients’ eligibility for participating voluntarily in this study was decided on the basis of preliminary health screening which included collection of individual's medical history, physical examination and primary diagnosis. All patients were enrolled for treatment after a written informed consent.
Immunophenotyping
Immunophenotyping of peripheral blood and/or bone marrow samples was performed by a four color flow cytometer (FACS Caliber, Becton Dickinson, San Jose, CA). Samples were processed using standard techniques.[4] Each case was evaluated with a panel of monoclonal antibodies (CD2, CD5, CD7, CD10, CD19, CD22, CD34 and CD45) and cases were assigned immunologic subtypes according to the classification of World Health Organization (WHO).
Hematological measurements
Routine hematological parameters like hemoglobin, WBC and platelet counts were done using a Sysmex semi-automatic analyzer.
Statistical analysis
The overall survival (OS), event free survival (EFS) and disease free survival (DFS) rates were calculated. Statistical analysis was performed using SPSS 15.0 (SPSS Inc., Chicago, IL, USA).
RESULTS
In the present population-based investigation, a sample population consisting of 500 consecutive ALL patients were selected, diagnosed and treated according to National Cancer Institute protocol (MCP 841) for a prolonged period of 24-88 months (with an average of 54 months) at NCRI, a tertiary care cancer institute in Eastern India.[5]
Majority of cases (50.4%; 252) in our sample population was characterized by a higher incidence of T-cell immunophenotype whereas, 47.6% (238) patients had pro-B, B-cell precursor ALL and the rest (2%; 10) were suffering from mature B-ALL. Predominance of T-ALL among adolescents was found having distinctive clinical and molecular features, about 60.2%, approximately twice that of pro-B or B-cell precursor ALL.
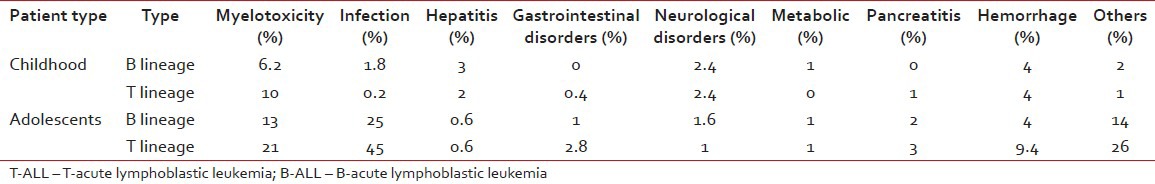
DFS (72.4%) and EFS (70.2%) were significantly better for B-ALL patients when all 500 patients were considered. For adolescents also, both DFS and EFS were higher in case of B-ALL than T-ALL [Tables [Tables11 and and2].2]. Patients with T-cell subtyping had a significantly lower OS ratio [Table 3]. Moreover, comparatively older patients (13-18 years) had relatively inferior outcome (61.33%) in contrast to total patients (73.5%). A difference in the pattern of relapse was also noted. 102 cases (around 20.4%) studied during diagnosis had an isolated relapse in contrast to 73 cases among older patients, resulting in a significantly higher relapse rate for adolescents [Table 4]. Toxicity of chemotherapy was also higher in adolescents and T-cell subtypes [Table 5].
Table 1
Comparison of DFS between T- and B-lineage ALL cases of children and adolescents

Table 2
Comparison of EFS between T- and B-lineage ALL cases of children and adolescents
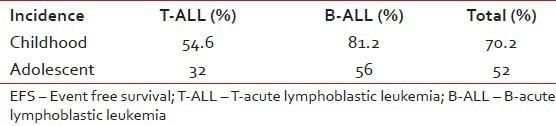
Table 3
Comparison of EFS between T- and B-lineage ALL cases of children and adolescents

Table 4
Comparison of relapse between T- and B-lineage ALL cases of children and adolescents

Table 5
Comparison of toxicity between T- and B-lineage ALL cases of children and adolescents
DISCUSSION
Published reports on incidence and subtypes of ALL among these patients based on WHO classification were few from India. In general, prognosis of childhood and adolescent ALL in the developing world remains poor due to a multitude of adverse clinical and social factors, the most prominent among these being the lack of resources available to both patients and health care professionals. We evaluated 500 consecutive new cases of ALL during this hospital-based study. T-lineage ALL accounted for 44.9% children and 60.2% adolescents. In the West, the predominant immunophenotype observed in ALL was B-ALL, accounting for 60-80% of total cases, whereas T-ALL comprised only of 15-20%.[6] In India also, studies reported from Tata Memorial Hospital, Mumbai, All India Institute of Medical Sciences, New Delhi and Cancer Institute, Chennai revealed a declining trend of T-ALL accompanied by an increasing trend in B-ALL among children.[7,8,9] Thus most of these studies have focused predominantly on B-ALL. Moreover, in the existing scenario, specific outcome data for adolescents was relatively sparse. With this in mind, in the present study, we evaluated the immunophenotypic patterns of ALL in Eastern India with special emphasis on patients of 13-18 years where ALL was relatively uncommon but had a worse outcome than younger children. Although our overall result demonstrated almost equal prevalence of B- or T-lymphoblastic leukemia, significant heterogeneity was noticed among cases of T-ALL that correlate with patient age. Again population-based studies of ALL in Eastern India are rare. We noticed a pattern of increased prevalence of T-ALL among adolescents in this region.
CONCLUSIONS
Our findings recommend that analysis of immunophenotypic subtypes can help to evaluate and predict the outcome of patients with ALL. Thus, this study may help planning for delivery of routine clinical care services and design of new age-focused treatments. We further plan to investigate the effect of socioeconomic and environmental factors in determining the major subtypes of pediatric ALL cases for a longer period of time. Thus, we can select the high risk adolescent and child ALL by aggressive chemotherapy protocol like Berlin-Frankfurt-Munich to render better outcome.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.


 PDF
PDF  Views
Views  Share
Share

