Terminal Deoxynucleotidyl Transferase (TdT) Positive Acute Myeloid Leukemia with C-MYC and BCL2 Expression: A Distinct Biological Entity with Potential Clinical Implications
CC BY 4.0 · Indian J Med Paediatr Oncol 2025; 46(05): 508-516
DOI: DOI: 10.1055/s-0045-1809173
Abstract
Terminal deoxynucleotidyl transferase (TdT) is a unique deoxyribonucleic acid polymerase whose overexpression has been used as an important biomarker in the diagnosis of precursor B or T cell acute lymphoblastic leukemia/lymphoma. Over the last few years, TdT has been increasingly implicated in the genesis of mutant Fms-like tyrosine kinase 3-internal tandem duplication (FLT3-ITD) and NPM1 isoforms, two key events in the myeloid stem cell leukemogenesis. This article studies the diagnostic and prognostic implications of immunoexpression of TdT, C-MYC, and BCL2 in acute myeloid leukemia (AML). We retrospectively reviewed seven cases of TdT positive (+), myeloperoxidase negative AMLs (2022–2024) with C-MYC and/or BCL2 expression by immunohistochemistry (IHC), and correlated these with flow cytometric (FC), molecular, and/or cytogenetics data (wherever available) along with outcome following induction chemotherapy. Morphologically, all these cases resembled French-American-British M0, M1, and M5 AMLs, or even high-grade lymphoproliferative neoplasms with moderate to stronger nuclear positivity for TdT and C-MYC in a higher proportion neoplastic cell along with stronger cytoplasmic BCL2 expression. Two were FLT3-ITD mutated, one was TP53 mutated, one had high-risk cytogenetics, whereas the remainder had no detectable abnormalities on molecular testing with variable degree of therapeutic response. Literature review highlighted the negative impact of overall, event-free, and relapse-free survivals among TdT and C-MYC positive AMLs whereas BCL2 expressors were likely to have increased chemoresistance. We suggest that TdT +/C-MYC +/BCL2+ AMLs may serve as distinct biological subtype; and IHC/FC-based testing should be a part of routine diagnostic workup for better risk stratification in AMLs.
Authors' Contributions
C.P. and S.P.: Conceptual design, data curation, diagnosis, writing the original draft, review, and editing. B.B., P.K.D., and A.P.: Management of cases, follow-up data, and critical review of scientific content. G.C., S.P., and D.J.: Flow cytometry evaluation of cases. S.S. and P.A.: Diagnosis and literature review. A.C.: Literature review.
Ethical Approval
The study was part of the retrospective review proposal approved by the Institutional Ethics Committee of AIIMS, Bhubaneswar (T/IM-NF/Patho/24/52).
Patient's Consent
Patient consent is not required.
Publication History
Article published online:
19 May 2025
© 2025. The Author(s). This is an open access article published by Thieme under the terms of the Creative Commons Attribution License, permitting unrestricted use, distribution, and reproduction so long as the original work is properly cited. (https://creativecommons.org/licenses/by/4.0/)
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India
- PAX5 and TDT-Negative B-Acute Lymphoblastic Leukemia with Unusual Genetic Mutations: A Case ReportTariq N. Aladily, Avicenna Journal of Medicine, 2022
- Precursor B-cell Lymphoblastic Lymphoma of Bone in Children: A Close Mimicker of Ewing's SarcomaShwetha Seetharam, Indian Journal of Medical and Paediatric Oncology, 2018
- Utility of Immunohistochemistry on Bone Marrow Trephine Biopsy for the Diagnosis and Classification of Acute LeukemiaH Sudha Rani, Indian Journal of Medical and Paediatric Oncology, 2020
- In-Situ End Labelling with Bromodeoxyuridine - an Advanced Technique for the Visualization of Apoptotic Cells in Histological SpecimensA. Aschoff, Hormone and Metabolic Research, 1996
- In-Situ End Labelling with Bromodeoxyuridine - an Advanced Technique for the Visualization of Apoptotic Cells in Histological SpecimensA. Aschoff, Hormone and Metabolic Research, 1996
- Resveratrol inhibits neuronal apoptosis and elevated Ca2+/calmodulin-dependent protein kinase II activity in diabetic mouse retina.<svg viewBox="0 0 24 24" fill="none" xmlns="http://www.w3.org/2000/svg">
- Imatinib Mesylate Reduces Endoplasmic Reticulum Stress and Induces Remission of Diabetes in db/db Mice<svg viewBox="0 0 24 24" fill="none" xmlns="http://www.w3.org/2000/svg">
- Molecular mechanisms of unique therapeutic potential of CUDC-907 for MEF2D fusion-driven BCP-ALL<svg viewBox="0 0 24 24" fill="none" xmlns="http://www.w3.org/2000/svg">
- Expression and regulation of neuronal apoptosis inhibitory protein during adipocyte differentiation.<svg viewBox="0 0 24 24" fill="none" xmlns="http://www.w3.org/2000/svg">
- Comment on Marcoux et al. Varying Impact of Gestational Diabetes Mellitus on Incidence of Childhood Cancers: An Age-Stratified Retrospective Cohort Study. Diabe...<svg viewBox="0 0 24 24" fill="none" xmlns="http://www.w3.org/2000/svg">
Abstract
Terminal deoxynucleotidyl transferase (TdT) is a unique deoxyribonucleic acid polymerase whose overexpression has been used as an important biomarker in the diagnosis of precursor B or T cell acute lymphoblastic leukemia/lymphoma. Over the last few years, TdT has been increasingly implicated in the genesis of mutant Fms-like tyrosine kinase 3-internal tandem duplication (FLT3-ITD) and NPM1 isoforms, two key events in the myeloid stem cell leukemogenesis. This article studies the diagnostic and prognostic implications of immunoexpression of TdT, C-MYC, and BCL2 in acute myeloid leukemia (AML). We retrospectively reviewed seven cases of TdT positive (+), myeloperoxidase negative AMLs (2022–2024) with C-MYC and/or BCL2 expression by immunohistochemistry (IHC), and correlated these with flow cytometric (FC), molecular, and/or cytogenetics data (wherever available) along with outcome following induction chemotherapy. Morphologically, all these cases resembled French-American-British M0, M1, and M5 AMLs, or even high-grade lymphoproliferative neoplasms with moderate to stronger nuclear positivity for TdT and C-MYC in a higher proportion neoplastic cell along with stronger cytoplasmic BCL2 expression. Two were FLT3-ITD mutated, one was TP53 mutated, one had high-risk cytogenetics, whereas the remainder had no detectable abnormalities on molecular testing with variable degree of therapeutic response. Literature review highlighted the negative impact of overall, event-free, and relapse-free survivals among TdT and C-MYC positive AMLs whereas BCL2 expressors were likely to have increased chemoresistance. We suggest that TdT +/C-MYC +/BCL2+ AMLs may serve as distinct biological subtype; and IHC/FC-based testing should be a part of routine diagnostic workup for better risk stratification in AMLs.
Keywords
immunohistochemistry - TdT - myeloid leukemia - molecular testing - prognosisIntroduction
Terminal deoxynucleotidyl transferase (TdT) is a nuclear template-independent deoxyribonucleic acid (DNA) polymerase that catalyzes the addition of deoxynucleotides to the 3′-hydroxyl terminus of oligonucleotide primers during V(D)J recombination to enhance antigen receptor diversity. Therefore, it plays a crucial role in the development of immature (precursor) B (pre-B) and T (pre-T) lymphoid cells in the bone marrow (BM) and thymus, respectively.[1] The template-independent insertion of nucleotide is unique for TdT that also makes it a potentially vulnerable target for neoplastic transformation of pre-B or pre-T lymphoid cells.[2] TdT overexpression is considered as an important biomarker in the diagnostic evaluation of pre-B/pre-T acute lymphoblastic leukemia/lymphoma (ALL), lymphoblastic blast crisis in chronic myeloid leukemia (CML-LB), and some non-Hodgkin lymphomas (NHLs).[3] Although TdT expression is primarily limited to lymphoid stem cells to restrict genetic infidelity, discovery of frequent TdT expression among myeloid stem cell lines has potentially increased the risk for development of acute myeloid leukemia (AML).[4] [5] In this article, we aimed to describe TdT expression in a series of AML cases at our center and comprehensively review the literature on TdT expression in AML and their possible interaction with other prognostic biomarkers such as C-MYC and BCl2.
Materials and Methods
We retrospectively reviewed all myeloperoxidase (MPO) negative AML cases (≥ 18 years) diagnosed and managed at our center over the last 2 years (April 2022–April 2024). The study was part of the retrospective record review and analyses cleared by the Institutional Ethics Committee (ref. no. T/IM-NF/Patho/24/52). All cases of AML were diagnosed and differentiated from ALL, early T-precursor ALL (ETP-ALL), and mixed phenotypic acute leukemia based upon constellation of morphologic (blast proportion, French-American-British [FAB] morphology, cytochemistry) and immunophenotypic characteristics supplemented with molecular/cytogenetics data (wherever available) as per the 2017 World Health Organization (WHO) guidelines.[6] MPO negativity was confirmed by cytochemistry and flow cytometric (FC) immunophenotypic analysis performed on either peripheral blood (PB) and/or BM aspirate (BMA) sample as well as on BM trephine biopsy (BMBx) sections using immunohistochemistry (IHC) (mentioned below). Immunohistochemical expression of TdT of any intensity (weak/dim [+], moderate [++], or strong [++ + ]) positivity in at least 10% myeloid blasts was considered as positive; and the same was correlated with FC [removed]wherever available) and other antigen expression profile. Additionally, TdT expression was corroborated with other markers such as CD10, BCL2, C-MYC, and cyclin D1 wherever performed to confirm or rule out B cell NHLs. All cases of acute promyelocytic leukemias, myelodysplastic syndrome-increased blast 2 (MDS-IB2), and CML in myeloid blast crisis (CML-MB) were excluded from the review. The clinicomorphological and immunophenotypic features were correlated with molecular and/or cytogenetics data (wherever available) and therapeutic outcome following induction chemotherapy (CT). The Institutional Review Board of our institute approved the retrospective study protocol.
Immunophenotyping
IHC was performed on paraffin-embedded, 14% ethylenediaminetetraacetic acid decalcified trephine biopsy sections following standard protocol and using following selected antibodies in variable combination on a case-to-case basis as follows: CD34 (Q-band 10, 1:100), CD117 (EP10, 1:100), MPO (EP151, ready to use, RTU), TdT (E266, RTU), BCl2 (EP36, 1:100), C-MYC (EP121, RTU), cyclin D1 (EP12, RTU) (all PathnSitu), and CD10 (DAKO, RTU). FC immunophenotypic analysis was performed on PB and/or BMA sample using FACS Canto II flow cytometer (BD Biosciences, India) using a panel of antibodies and appropriate fluorochromes (presented in [Table 1]). The sample was processed using the stain-lyse-wash procedure. Initially, the sample was stained with an antibody panel consisting of pre-titrated antibodies for surface staining followed by the fixation, permeabilization, and staining for nuclear (Nu) TdT antibody (APC, clone E-17–1519, BD Biosciences, San Jose, California, United States).
|
Fluorochromes |
V450 |
V500c |
FITC |
PE |
PerCPCy5.5 |
PECy7 |
APC |
APCH7 |
|---|---|---|---|---|---|---|---|---|
|
A LOT |
cyCD3 |
CD45 |
cyMPO |
cyCD79a |
CD34 |
CD19 |
CD7 |
smCD3 |
|
B-tube |
CD20 |
CD45 |
CD58 |
CD66c |
CD34 |
CD19 |
CD10 |
CD38 |
|
AML tube |
HLA-DR |
CD45 |
CD64 |
CD13 |
CD34 |
CD117 |
CD33 |
CD14 |
|
T tube |
CD4 |
CD45 |
CD1a |
– |
CD5 |
CD2 |
nuTdT |
CD8 |
|
AML extra tube |
CD45 |
CD15 |
CD56 CD61 |
CD34 CD11c |
CD41a |
CD123 |
CD71 |
Abbreviations: AML, acute lymphoblastic leukemia; APC, adenomatous polyposis coli; FITC, fluorescein isothiocyanate; PE, phycoerythrin.
a FACS Canto II flow cytometer (BD Biosciences, India).
Cytogenetics and/or Molecular Studies
Therapy and Monitoring
|
Sl. No. |
Age, y, gender |
Presentation |
Peripheral blood smear |
Bone marrow blasts, FAB morphology |
TdT/ intensity[a] |
FC intensity |
C-MYC (%), intensity[a] |
BCL2 (%), intensity[a] |
Molecular/cytogenetics |
Follow-up |
|---|---|---|---|---|---|---|---|---|---|---|
|
1 |
21, male |
Bone pain, fatigue |
Pancytopenia, 9% blast |
95%, M0/M1 |
90%, +++ |
CD342+ CD1172+ TdTNP MPO− CD2+ CD19+ |
20%, ++ |
Not done |
FLT/ITD; mutated by FISH |
Remission, lost to follow-up |
|
2 |
18, male |
Weakness, lethargy |
A + T with 70% blast |
90%, M1/M5 |
90%, +++ |
CD342+ CD1172+ MPO−/+ TdTNP CD19+ |
– |
30%, ++ |
FLT/ITD; mutated NF1; mutated PHF6; mutated (by FISH) |
Remission |
|
3 |
61, male |
Weakness, fever, k/c/o tuberculosis on ATT |
A + L with 3% blast |
50% (AML-MR), M0 |
60%, +++ |
CD34+ CD117+ HLA-DR+ MPO− CD13+ TdT2+ CD7 + (aberrant) |
Not done |
20%, ++ |
46, XY; t(6;17) (q15;p13), t(4;8) (q31.3;q24.1) FISH; negative |
Expired |
|
4 |
32, female |
Heavy menstrual bleed |
Pancytopenia |
50%, M4/M5 |
60%, ++ |
CD34+ CD117+ MPO+ TdTNP CD79a+ |
Not done |
Not done |
Del 17q; positive (by NGS) |
Partial remission, lost to follow-up |
|
5 |
73, male |
Coronary artery disease |
Pancytopenia with 35% blast |
70%, M0/M1 |
10%, ++ |
CD342+ CD117+ MPO+ TdT+ |
30%, +++ |
Not done |
No fusion identified (by NGS) |
Lost to follow-up |
|
6 |
57, male |
Fatigue |
A + L with lymphoplasmacytoid cells, platelet; 300 × 109/L Rouleaux present; ?LPL/WM |
60%, M0/M5/PLL |
70%, +++ |
CD342+ TdT+++ CD1172+ HLA- DR+++ MPO+/− CD19− CD20− |
25%, ++ |
70%, +++ |
No fusion identified (by NGS) |
Remission, alive |
|
7 |
17, male |
Breathlessness, lymphadenopathy, pleural effusion |
A + T Marked leukocytosis (500 × 109/L) > 95% blasts ETP-ALL |
> 90% undifferentiated blasts; FAB M0 > ETP-ALL |
15%, ++ |
CD34+++ CD117+++ HLA-DR2+ TdT+ MPO− CD13+++ CD332+ cyCD3− CD1a− CD4−CD8− CD5− CD7+++ CD19− CD79a+ |
60%, ++ |
80%, +++ |
No fusion identified (by NGS) |
Remission, alive |
a On trephine biopsy immunohistochemistry (IHC).
Results
The clinicopathological, immunophenotypic, and molecular data of seven cases of TdT positive AML are presented in [Table 2]. These constituted 11% of 64 adult AMLs diagnosed during the last 2 years. These included six males and one female with a median age of 32 years (range: 17–73) at diagnosis. One of the cases (no. 7) presented with breathlessness, generalized lymphadenopathy, and marked leukocytosis (500 × 109/L), thus simulating clinically a lymphoma/leukemia such as ALL. Peripheral smear and BMA examination revealed mostly undifferentiated blasts that resembled FAB M0, M1, or M5 phenotype or a high-grade lymphoproliferative neoplasm; prominent rouleaux formation with presence of lymphoplasmacytoid lymphoid cells in presence of adequate platelets resembled that of lymphoplasmacytic lymphoma/Waldenstrom macroglobulinemia in another (no. 6); and of ETP-ALL phenotype with prominent cytoplasmic blebbing in case no. 7. In case no. 3, the BM morphology was consistent with AML-myelodysplasia-related (AML-MR, WHO 2022) where it resembled that of a prolymphocytic leukemia in another (case no. 6). Trephine biopsy section showed an interstitial to diffuse marrow infiltration pattern (blast percentage; 50–95%) in all with focal, myelofibrosis 1 to 2 reticulin (WHO) fibrosis.
On IHC, intermediate to strong, homogenous, nuclear TdT immunopositivity was noted in all cases with a TdT positive blast proportion ranging from 10 to 90% (median: 60%), whereas FC immunoexpression was variable along with variable intensity aberrant expression of B and T lineage markers. Moreover, C-MYC (nuclear) and BCL2 (cytoplasmic), performed in 4/7 cases, showed variable intensity positivity in 20 to 70% of blasts ([Figs. 1] [2] [3] [4] [5] [6]). Molecular/cytogenetic analyses revealed mutated FLT3/ITD in two, translocation t(6;17) (q15;p13) and t(4;8) (q31.3;q24.1) in one with AML-MR, del17q in one, whereas no abnormalities were identified in either FISH- or NGS-based studies in the remainder of the cases. Mutated NPM1 was not identified in any of these cases. Three achieved complete remissions, one expired, and the remaining two were lost to follow-up after in remission.
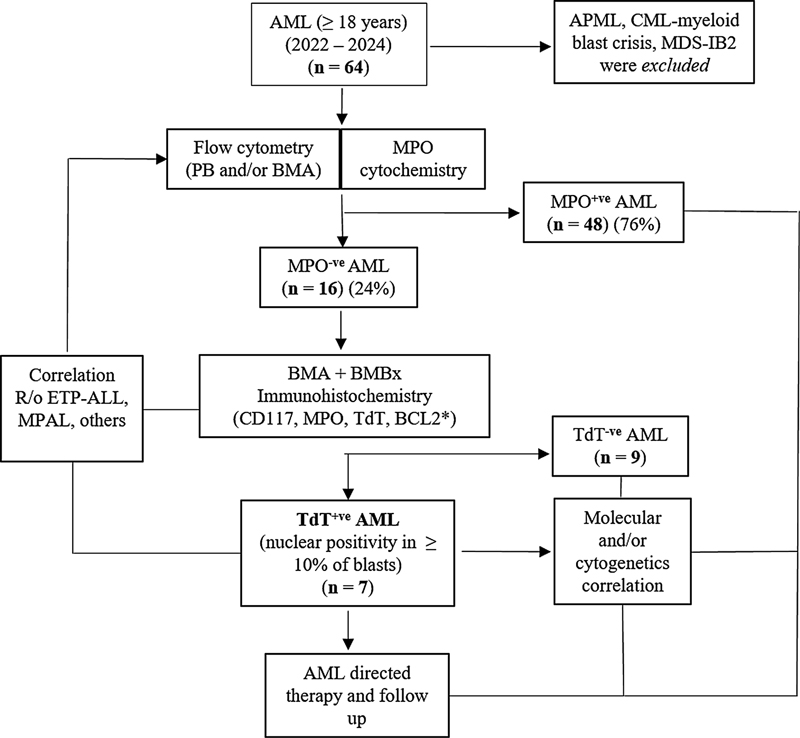
Fig 1: Flowchart 1. The stepwise evaluation of all cases of terminal deoxynucleotidyl transferase (TdT) positive acute myeloid leukemia cases among adults (≥ 18 years) (2022–2024) at our center.
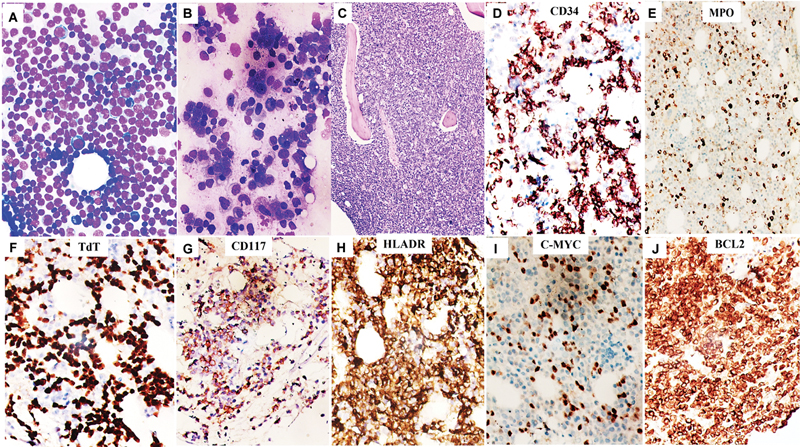
Fig 2 : Leishman–Giemsa stained hypercellular bone marrow aspirate smears from iliac crest at two different occasions at baseline (A, B) from a 57-year-old male with acute leukemia with undifferentiated morphology mimicking a lymphoproliferative neoplasm, and trephine biopsy sections showing a packed marrow with total replacement by lesional cells (C, hematoxylin and eosin). On immunohistochemistry (IHC), these cells were positive for CD34 (strong, D), myeloperoxidase (MPO) (weak, E), TdT (strong, F), CD117 (weak, focal, G), HLA-DR (strong, H), C-MYC (strong, I), and BCL2 (strong, J), consistent with TdT + acute myeloid leukemia (AML) (all magnifications ×400).
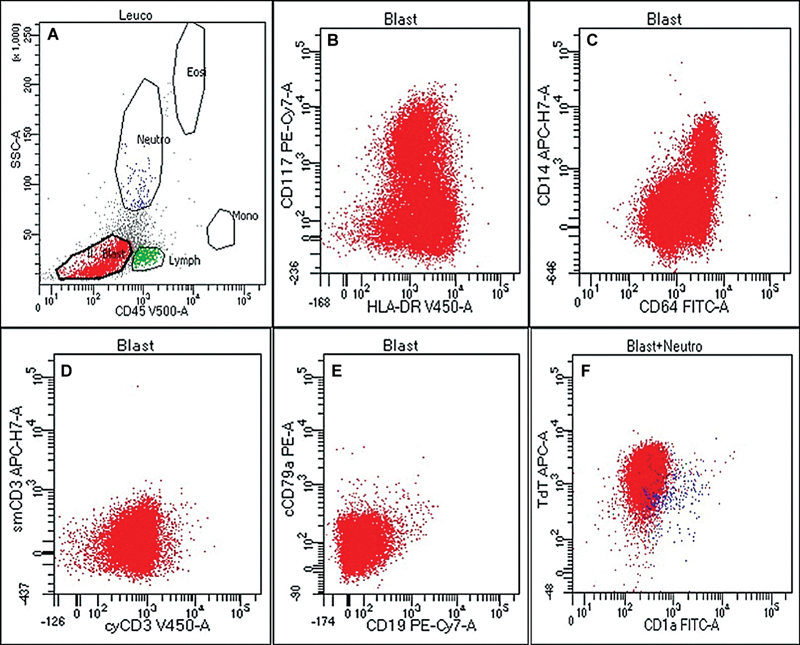
Fig 3 : Flow cytometric immunophenotyping on second bone marrow aspirate sample from the above case ([Fig. 2]) depicting gated population of abnormal cells (dim to negative CD45 vs. low-intermediate side scatter [SSC], blast, A), which were positive for CD117 (heterogeneous) and HLA-DR (bright) (B), CD64 and CD14 (C), negative for T (D) and B (E) cell markers, and positive for nuclear terminal deoxynucleotidyl transferase (nuTdT) (F).
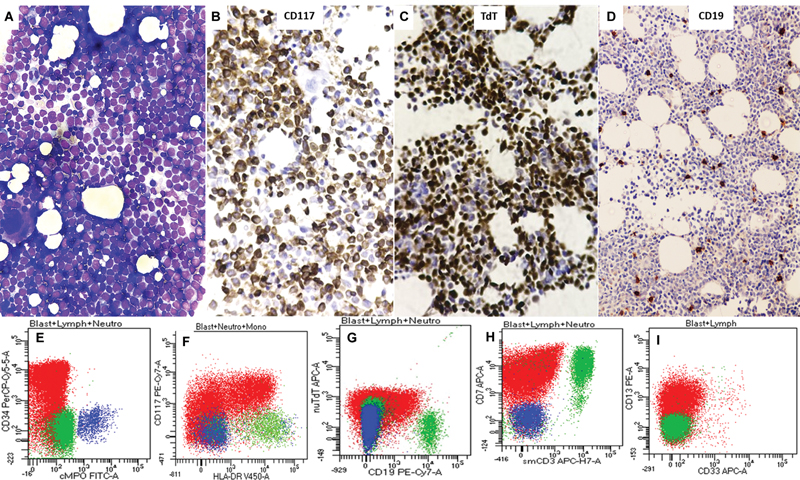
Fig 4 : Bone marrow aspirate smear from a 61-year-old male with worsening pancytopenia showing sheets of highly undifferentiated neoplastic cells resembling high grade lymphoproliferative neoplasm or even, leukemia (A). On trephine biopsy and IHC, these cells were strongly and diffusely positive for CD117 (B) and terminal deoxynucleotidyl transferase (TdT) (C), negative for myeloperoxidase (MPO) (not shown), and CD19 (D). Original magnification, ×400 (A–C) (×200, D). Note the corresponding flow cytometric immunophenotyping panel (E to I) depicting dim expression of nuTdT (G) from the same case [blasts (in red) are positive for CD34 (E), CD117 (F), CD7 (H, aberrant), and CD13 (I, heterogeneous)].
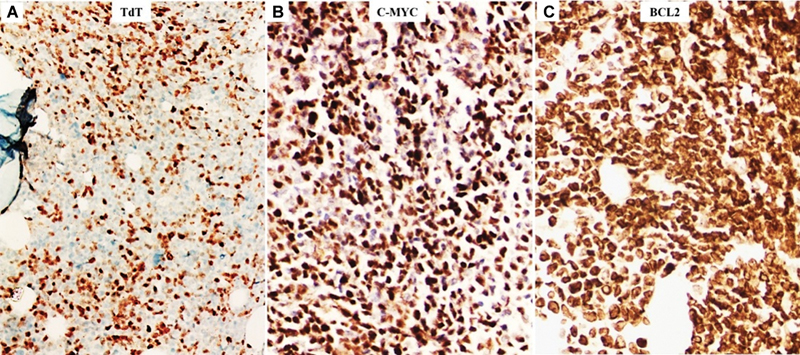
Fig 5 : Immunohistochemical expression of terminal deoxynucleotidyl transferase (TdT) (A), C-MYC (B), and BCL2 (C) from an adolescent male (case 7, [Table 1]) with generalized lymphadenopathy and high leukocytosis clinically and morphologically suspected to be that of lymphoma/leukemia (possibly early T-precursor acute lymphoblastic leukemia [ETP-ALL]) (×400).
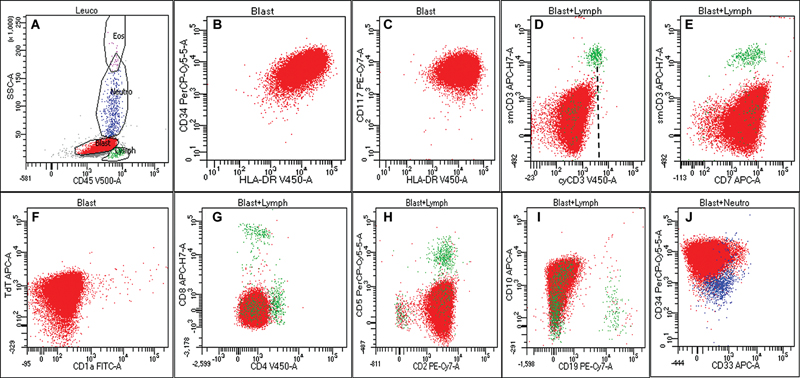
Fig 6 : Flow cytometric (FC) immunophenotypic analysis of peripheral blood sample from the above case ([Fig. 5] (Case 7)) showing gated population of cells (dim to moderate CD45 vs. low side scatter [SSC], A) immunopositive for CD34, HLA-DR (B), CD117 (bright, C), CD7 (E), nuclear terminal deoxynucleotidyl transferase (nuTdT) (F), CD2 (H), and CD10 (I). The lesional cells were negative for cytoCD3 (D, dotted black line representing the control T cell population), CD1a (F), both CD4 and CD8 (G), CD5 (H), and other B cell markers. Although the cyCD3 expression pattern did reach up to the control population (green), (D) the possibility of early T-precursor acute lymphoblastic leukemia (ETP-ALL) was ruled out by negative CD3 expression on immunohistochemistry (IHC) (not shown) as well as on repeat flow cytometry evaluation on fresh marrow aspirate sample.
Discussion
Borrow et al in their 2019 cutting edge work have highlighted the pivotal role of TdT in both pediatric and adult myeloid leukemia stem cell lines.[3] [4] They have tried to shed more light into the underlying molecular events that lead to the generation and accumulation of mutated FLT3-ITD and NPM1; two important driver mutations that facilitate risk-adapted therapy in AML. It was proven in TdT models that illegitimate insertional activity of TdT at the 3′ end was the main factor behind the priming of replication slippage of both FLT3-ITD and NPM1 molecules producing more mutated forms in nearly half of all AMLs.[3] [4] [5] Further reports also suggested significantly higher TdT expression among t(6;9) DEK-NUP214 (with marrow basophilia and myelodysplasia) and t(5;11) NUP98-NSD1 fusion (AML in children with FAB M4/M5 phenotype), but uncommonly with t(8;21) RUNX1/RUNX1T1 and inv16 AMLs, which are more likely to harbor FLT3-ITD mutations and conferring a poor prognosis.[9] [10] Moreover, TdT expression was also reported to be linked to the accumulation of mutated FLT3-ITD in variant acute promyelocytic leukemia (M3v) with aberrant lymphoid antigenic expression than the classical/hypergranular phenotype.[11]
Literature on the diagnostic and prognostic implications of TdT expression in AML is sparse.[12] [13] [14] Shaforostova et al,[12] using FC, reported cytoplasmic TdT (cyTdT) positivity among ≥ 20% blasts among 19 of 324 (6%) adult AMLs (median age: 67 years), which was linked to aberrant CD2 co[removed]p < 0.05), lesser incidence of mutated FLT3-ITD (p = 0.52), but wild-type NPM1 (p = 0.04). However, cyTdT and CD2 coexpression was associated with inferior overall survival (OS) (log rank p = 0.10), relapse-free survival (RFS) (p = 0.037), and 3-year event-free survival (EFS) (p = 0.003).[12] De Bellis et al[13] supported Burrow's models where TdT positivity was reported by FC analysis among 49 of 143 (34.2%) de novo AML subjects (62.5 years, range: 21–86 years) and this was linked to male gender (p = 0.078), mutated FLT3-ITD subgroups (p = 0.035), and an inferior 5-year OS (log rank p = 0.03).[13] Another Japanese group reported a significantly inferior 1-year RFS (hazard ratio: 3.309, 95% confidence interval: 1.334–8.209, p = 0.009) among TdT positive (12/45, 25%) (≥ 10% blasts by FC) de novo AMLs (68 years, range: 16–87) with intermediate-risk karyotype.[14]
Interestingly, we reported significant proportion of myeloid blasts with C-MYC and BCL2 overexpression in some of our TdT positive AML cases. The largest data from MD Anderson Cancer Center[15] tried to evaluate the prognostic impact of C-MYC [removed]moderate to strong nuclear positivity among ≥ 3% myeloid blasts by IHC) among 265 newly diagnosed AMLs (median age: 63 years, range: 22–88, male-to-female: 1.15:1) diagnosed over a period of 7 years. C-MYC expression was reported in a higher proportion (238/265, 90%, de novo > secondary AMLs, p = 0.012) of cases with mean percentage of blast positivity of 32%. A cutoff ≥ 6% blasts with C-MYC positivity was associated with shorter complete response duration (p = 0.007), inferior OS (log rank p = 0.042), EFS (p = 0.048), and RFS (p = 0.024) among older (≥ 55 years) subjects and those with intermediate-high risk cytogenetics.[15] Another study by Gajzer et al[16] demonstrated that de novo MDS subjects with high MYC [removed]in ≥ 5% myeloid blasts by IHC) (N = 16/41, 39%) had significantly shorter OS (19.7 vs. 51.7 months, p = 0.053) and MDS-to-AML progression-free survival (median: 9.3 vs. 17.7 months, respectively, p = 0.013) than low expressors; and high MYC expression was an independent prognostic indicator for MDS-to-AML progression in multivariate regression analysis (hazard ratio = 2.275, p = 0.046).[16]
Overexpression of BCL2 group of antiapoptotic proteins in AMLs has been increasingly reported to be associated with increased chemoresistance and inferior survival.[17] [18] In 2017, discovery of venetoclax, a BCL2 inhibitor, led to a major breakthrough in AML-CT; and this in combination with azacitidine, decitabine, or low-dose cytarabine (LDAC) got Food and Drug Administration approval in 2018 for the use in elderly (≥ 75 year) AMLs as well as those found unfit for intensive induction CT.[18]
To summarize, we described seven adult AML cases with TdT expression using IHC with correlation with flow cytometry, molecular, and follow-up details. IHC is a simple and cost-effective diagnostic tool to evaluate the immunoexpression of TdT, C-MYC, and BCL2 on BMBxs in cases with newly diagnosed AMLs. Available and emerging new evidences point to an increased prognostic impact of such biomarkers in risk stratification and therapy in AMLs, which most likely involve several key players like FLT3-ITD and NPM1. Incorporation of such vital prognostic information into the routine hematopathology is necessary for devising better therapeutic strategies in AML and MDS cases.
Conflict of Interest
None declared.
Acknowledgments
The authors sincerely thank Dr. Pavithra Ayyanar, Associate Professor, Department of Pathology and Laboratory Medicine, All India Institute of Medical Sciences, for her intellectual inputs on the study.
Authors' Contributions
C.P. and S.P.: Conceptual design, data curation, diagnosis, writing the original draft, review, and editing. B.B., P.K.D., and A.P.: Management of cases, follow-up data, and critical review of scientific content. G.C., S.P., and D.J.: Flow cytometry evaluation of cases. S.S. and P.A.: Diagnosis and literature review. A.C.: Literature review.
Ethical Approval
The study was part of the retrospective review proposal approved by the Institutional Ethics Committee of AIIMS, Bhubaneswar (T/IM-NF/Patho/24/52).
Patient's Consent
Patient consent is not required.
References
- Motea EA, Berdis AJ. Terminal deoxynucleotidyl transferase: the story of a misguided DNA polymerase. Biochim Biophys Acta 2010; 1804 (05) 1151-1166
- Patel KP, Khokhar FA, Muzzafar T. et al. TdT expression in acute myeloid leukemia with minimal differentiation is associated with distinctive clinicopathological features and better overall survival following stem cell transplantation. Mod Pathol 2013; 26 (02) 195-203
- Borrow J, Dyer SA, Akiki S, Griffiths MJ. Terminal deoxynucleotidyl transferase promotes acute myeloid leukemia by priming FLT3-ITD replication slippage. Blood 2019; 134 (25) 2281-2290
- Borrow J, Dyer SA, Akiki S, Griffiths MJ. Molecular roulette: nucleophosmin mutations in AML are orchestrated through N-nucleotide addition by TdT. Blood 2019; 134 (25) 2291-2303
- Vassiliou GS. The curious incident of TdT-mediated mutations in AML. Blood 2019; 134 (25) 2229-2231
- Arber DA, Orazi A, Hasserjian R. et al. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood 2016; 127 (20) 2391-2405
- Pollyea DA, Altman JK, Assi R. et al. Acute myeloid leukemia, version 3.2023, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw 2023; 21 (05) 503-513
- Heuser M, Ofran Y, Boissel N. et al; ESMO Guidelines Committee. Electronic address: clinicalguidelines@esmo.org. Acute myeloid leukaemia in adult patients: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2020; 31 (06) 697-712
- Akiki S, Dyer SA, Grimwade D. et al. NUP98-NSD1 fusion in association with FLT3-ITD mutation identifies a prognostically relevant subgroup of pediatric acute myeloid leukemia patients suitable for monitoring by real time quantitative PCR. Genes Chromosomes Cancer 2013; 52 (11) 1053-1064
- Oyarzo MP, Lin P, Glassman A, Bueso-Ramos CE, Luthra R, Medeiros LJ. Acute myeloid leukemia with t(6;9)(p23;q34) is associated with dysplasia and a high frequency of FLT3 gene mutations. Am J Clin Pathol 2004; 122 (03) 348-358
- Takenokuchi M, Kawano S, Nakamachi Y. et al. FLT3/ITD associated with an immature immunophenotype in PML-RARα leukemia. Hematol Rep 2012; 4 (04) e22
- Shaforostova I, Call S, Evers G. et al. Prevalence and clinical impact of CD56 and T-cell marker expression in acute myeloid leukaemia: a single-centre retrospective analysis. eJHaem 2023; 5 (01) 93-104
- De Bellis E, Ottone T, Mercante L. et al. Terminal deoxynucleotidyl transferase (TdT) expression is associated with FLT3-ITD mutations in acute myeloid leukemia. Leuk Res 2020; 99: 106462
- Saburi M, Ogata M, Satou T. et al. Prognostic implications of TdT expression in acute myeloid leukemia with an intermediate-risk karyotype. Int J Hematol 2020; 112 (01) 17-23
- Ohanian M, Rozovski U, Kanagal-Shamanna R. et al. MYC protein expression is an important prognostic factor in acute myeloid leukemia. Leuk Lymphoma 2019; 60 (01) 37-48
- Gajzer D, Logothetis CN, Sallman DA. et al. MYC overexpression is associated with an early disease progression from MDS to AML. Leuk Res 2021; 111: 106733
- Wei Y, Cao Y, Sun R. et al. Targeting Bcl-2 proteins in acute myeloid leukemia. Front Oncol 2020; 10: 584974
- Tiribelli M, Michelutti A, Cavallin M. et al. BCL2 expression in AML patients over 65 years: impact on outcomes across different therapeutic strategies. J Clin Med 2021; 10 (21) 5096
Address for correspondence
Publication History
Article published online:
19 May 2025
© 2025. The Author(s). This is an open access article published by Thieme under the terms of the Creative Commons Attribution License, permitting unrestricted use, distribution, and reproduction so long as the original work is properly cited. (https://creativecommons.org/licenses/by/4.0/)
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India
- PAX5 and TDT-Negative B-Acute Lymphoblastic Leukemia with Unusual Genetic Mutations: A Case ReportTariq N. Aladily, Avicenna Journal of Medicine, 2022
- Precursor B-cell Lymphoblastic Lymphoma of Bone in Children: A Close Mimicker of Ewing's SarcomaShwetha Seetharam, Indian Journal of Medical and Paediatric Oncology, 2018
- Utility of Immunohistochemistry on Bone Marrow Trephine Biopsy for the Diagnosis and Classification of Acute LeukemiaH Sudha Rani, Indian Journal of Medical and Paediatric Oncology, 2020
- In-Situ End Labelling with Bromodeoxyuridine - an Advanced Technique for the Visualization of Apoptotic Cells in Histological SpecimensA. Aschoff, Hormone and Metabolic Research, 1996
- In-Situ End Labelling with Bromodeoxyuridine - an Advanced Technique for the Visualization of Apoptotic Cells in Histological SpecimensA. Aschoff, Hormone and Metabolic Research, 1996
- Molecular mechanisms of unique therapeutic potential of CUDC-907 for MEF2D fusion-driven BCP-ALL<svg viewBox="0 0 24 24" fill="none" xmlns="http://www.w3.org/2000/svg">
- Src inhibition potentiates MCL-1 antagonist activity in acute myeloid leukemia<svg viewBox="0 0 24 24" fill="none" xmlns="http://www.w3.org/2000/svg">
- A novel peptide 66CTG stabilizes Myc proto-oncogene protein to promote triple-negative breast cancer growth<svg viewBox="0 0 24 24" fill="none" xmlns="http://www.w3.org/2000/svg">
- The deacetylases HDAC1/HDAC2 control JAK2V617F-STAT signaling through the ubiquitin ligase SIAH2<svg viewBox="0 0 24 24" fill="none" xmlns="http://www.w3.org/2000/svg">
- Overexpressing natural killer group 2 member A drives natural killer cell exhaustion in relapsed acute myeloid leukemia<svg viewBox="0 0 24 24" fill="none" xmlns="http://www.w3.org/2000/svg">

Fig 1: Flowchart 1. The stepwise evaluation of all cases of terminal deoxynucleotidyl transferase (TdT) positive acute myeloid leukemia cases among adults (≥ 18 years) (2022–2024) at our center.

Fig 2 : Leishman–Giemsa stained hypercellular bone marrow aspirate smears from iliac crest at two different occasions at baseline (A, B) from a 57-year-old male with acute leukemia with undifferentiated morphology mimicking a lymphoproliferative neoplasm, and trephine biopsy sections showing a packed marrow with total replacement by lesional cells (C, hematoxylin and eosin). On immunohistochemistry (IHC), these cells were positive for CD34 (strong, D), myeloperoxidase (MPO) (weak, E), TdT (strong, F), CD117 (weak, focal, G), HLA-DR (strong, H), C-MYC (strong, I), and BCL2 (strong, J), consistent with TdT + acute myeloid leukemia (AML) (all magnifications ×400).

Fig 3 : Flow cytometric immunophenotyping on second bone marrow aspirate sample from the above case ([Fig. 2]) depicting gated population of abnormal cells (dim to negative CD45 vs. low-intermediate side scatter [SSC], blast, A), which were positive for CD117 (heterogeneous) and HLA-DR (bright) (B), CD64 and CD14 (C), negative for T (D) and B (E) cell markers, and positive for nuclear terminal deoxynucleotidyl transferase (nuTdT) (F).

Fig 4 : Bone marrow aspirate smear from a 61-year-old male with worsening pancytopenia showing sheets of highly undifferentiated neoplastic cells resembling high grade lymphoproliferative neoplasm or even, leukemia (A). On trephine biopsy and IHC, these cells were strongly and diffusely positive for CD117 (B) and terminal deoxynucleotidyl transferase (TdT) (C), negative for myeloperoxidase (MPO) (not shown), and CD19 (D). Original magnification, ×400 (A–C) (×200, D). Note the corresponding flow cytometric immunophenotyping panel (E to I) depicting dim expression of nuTdT (G) from the same case [blasts (in red) are positive for CD34 (E), CD117 (F), CD7 (H, aberrant), and CD13 (I, heterogeneous)].

Fig 5 : Immunohistochemical expression of terminal deoxynucleotidyl transferase (TdT) (A), C-MYC (B), and BCL2 (C) from an adolescent male (case 7, [Table 1]) with generalized lymphadenopathy and high leukocytosis clinically and morphologically suspected to be that of lymphoma/leukemia (possibly early T-precursor acute lymphoblastic leukemia [ETP-ALL]) (×400).

Fig 6 : Flow cytometric (FC) immunophenotypic analysis of peripheral blood sample from the above case ([Fig. 5] (Case 7)) showing gated population of cells (dim to moderate CD45 vs. low side scatter [SSC], A) immunopositive for CD34, HLA-DR (B), CD117 (bright, C), CD7 (E), nuclear terminal deoxynucleotidyl transferase (nuTdT) (F), CD2 (H), and CD10 (I). The lesional cells were negative for cytoCD3 (D, dotted black line representing the control T cell population), CD1a (F), both CD4 and CD8 (G), CD5 (H), and other B cell markers. Although the cyCD3 expression pattern did reach up to the control population (green), (D) the possibility of early T-precursor acute lymphoblastic leukemia (ETP-ALL) was ruled out by negative CD3 expression on immunohistochemistry (IHC) (not shown) as well as on repeat flow cytometry evaluation on fresh marrow aspirate sample.
References
- Motea EA, Berdis AJ. Terminal deoxynucleotidyl transferase: the story of a misguided DNA polymerase. Biochim Biophys Acta 2010; 1804 (05) 1151-1166
- Patel KP, Khokhar FA, Muzzafar T. et al. TdT expression in acute myeloid leukemia with minimal differentiation is associated with distinctive clinicopathological features and better overall survival following stem cell transplantation. Mod Pathol 2013; 26 (02) 195-203
- Borrow J, Dyer SA, Akiki S, Griffiths MJ. Terminal deoxynucleotidyl transferase promotes acute myeloid leukemia by priming FLT3-ITD replication slippage. Blood 2019; 134 (25) 2281-2290
- Borrow J, Dyer SA, Akiki S, Griffiths MJ. Molecular roulette: nucleophosmin mutations in AML are orchestrated through N-nucleotide addition by TdT. Blood 2019; 134 (25) 2291-2303
- Vassiliou GS. The curious incident of TdT-mediated mutations in AML. Blood 2019; 134 (25) 2229-2231
- Arber DA, Orazi A, Hasserjian R. et al. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood 2016; 127 (20) 2391-2405
- Pollyea DA, Altman JK, Assi R. et al. Acute myeloid leukemia, version 3.2023, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw 2023; 21 (05) 503-513
- Heuser M, Ofran Y, Boissel N. et al; ESMO Guidelines Committee. Electronic address: clinicalguidelines@esmo.org. Acute myeloid leukaemia in adult patients: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2020; 31 (06) 697-712
- Akiki S, Dyer SA, Grimwade D. et al. NUP98-NSD1 fusion in association with FLT3-ITD mutation identifies a prognostically relevant subgroup of pediatric acute myeloid leukemia patients suitable for monitoring by real time quantitative PCR. Genes Chromosomes Cancer 2013; 52 (11) 1053-1064
- Oyarzo MP, Lin P, Glassman A, Bueso-Ramos CE, Luthra R, Medeiros LJ. Acute myeloid leukemia with t(6;9)(p23;q34) is associated with dysplasia and a high frequency of FLT3 gene mutations. Am J Clin Pathol 2004; 122 (03) 348-358
- Takenokuchi M, Kawano S, Nakamachi Y. et al. FLT3/ITD associated with an immature immunophenotype in PML-RARα leukemia. Hematol Rep 2012; 4 (04) e22
- Shaforostova I, Call S, Evers G. et al. Prevalence and clinical impact of CD56 and T-cell marker expression in acute myeloid leukaemia: a single-centre retrospective analysis. eJHaem 2023; 5 (01) 93-104
- De Bellis E, Ottone T, Mercante L. et al. Terminal deoxynucleotidyl transferase (TdT) expression is associated with FLT3-ITD mutations in acute myeloid leukemia. Leuk Res 2020; 99: 106462
- Saburi M, Ogata M, Satou T. et al. Prognostic implications of TdT expression in acute myeloid leukemia with an intermediate-risk karyotype. Int J Hematol 2020; 112 (01) 17-23
- Ohanian M, Rozovski U, Kanagal-Shamanna R. et al. MYC protein expression is an important prognostic factor in acute myeloid leukemia. Leuk Lymphoma 2019; 60 (01) 37-48
- Gajzer D, Logothetis CN, Sallman DA. et al. MYC overexpression is associated with an early disease progression from MDS to AML. Leuk Res 2021; 111: 106733
- Wei Y, Cao Y, Sun R. et al. Targeting Bcl-2 proteins in acute myeloid leukemia. Front Oncol 2020; 10: 584974
- Tiribelli M, Michelutti A, Cavallin M. et al. BCL2 expression in AML patients over 65 years: impact on outcomes across different therapeutic strategies. J Clin Med 2021; 10 (21) 5096


 PDF
PDF  Views
Views  Share
Share

