The fight against cancer: Is it worthwhile?
CC BY-NC-ND 4.0 · Indian J Med Paediatr Oncol 2015; 36(02): 85-86
DOI: DOI: 10.4103/0971-5851.158833
Abstract
This article alludes to the findings of Tomasetti and Vogelstein and argues that for clinicians and scientists no matter how difficult understanding the pathogenesis of cancer may be, they remain the only hope for patients suffering from the disease. Data citing wide differences in cancer incidence in different parts of the world is presented to drive home the point that ′Bad luck′ is not a good enough explanation for cancer pathogenesis. There remains a lot to be uncovered in cancer and clinicians and scientists should strive to this end.
Publication History
Article published online:
12 July 2021
© 2015. Indian Society of Medical and Paediatric Oncology. This is an open access article published by Thieme under the terms of the Creative Commons Attribution-NonDerivative-NonCommercial-License, permitting copying and reproduction so long as the original work is given appropriate credit. Contents may not be used for commercial purposes, or adapted, remixed, transformed or built upon. (https://creativecommons.org/licenses/by-nc-nd/4.0/.)
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India
Abstract
This article alludes to the findings of Tomasetti and Vogelstein and argues that for clinicians and scientists no matter how difficult understanding the pathogenesis of cancer may be, they remain the only hope for patients suffering from the disease. Data citing wide differences in cancer incidence in different parts of the world is presented to drive home the point that ‘Bad luck’ is not a good enough explanation for cancer pathogenesis. There remains a lot to be uncovered in cancer and clinicians and scientists should strive to this end.
If there is one disease that has plagued the human race for centuries, captured the imaginations of writers and cinematographers, and to an extent, “foxed” clinicians and scientists — it has to be cancer. While President Nixon may have given this “war” a political twist in 1971, every single day oncologists and researchers go to work knowing that this is no easy battle. Three decades on and we are as yet unsure if we are winning the “war” or worse still, if we are fighting a losing battle.[1] Several large scale consortium based efforts has detailed alterations underlying the cancer genome at different time points and stages of the disease. However, the fundamental question that eludes is “what triggers off these changes?”
In a recent report Tomasetti and Vogelstein have presented an alternative perspective on the risk for cancer development.[2] The authors defined a new terminology, “extra risk score,” a product of lifetime risk and the total number of stem cell divisions (log10 values) within a specific tissue. Based on the extra risk score, they divided 31 of the studied cancers into those with a high score (D or deterministic) and low score (R or replicative). Intriguingly, Tomasetti and Vogelstein infer from their analysis that the trigger for 22 sporadic cancers is a random event that may just be due to plain old “bad luck.”[2] In addition, they also note role of certain aetiological factors in 9 cancers to which clear associations could be derived — most of which were well known hereditary cancers or cancers arising from infections, such as hepatocellular carcinoma in patients with chronic hepatitis C infections. The authors concluded that based on this, primary prevention would be applicable to D cancers but not R.
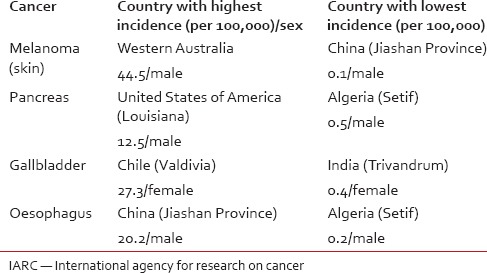
Most cancers are curable when detected at early stages. Of the 22 cancers with random development brings forth a concerning thought — how do we prevent or detect cancers early in the course if we don’t even know why they developed in the first place! Screening has been a tool employed to detect cancers, when they are asymptomatic (and thus hopefully in an early stage) in apparently healthy subjects. The whole process is labour intensive and thus is justifiable only in populations where the incidence of the disease is abnormally high. If the randomness in cancer development is true, then it certainly make us rethink the benefit of screening for all cancers, except for those cancers in areas of high disease prevalence, despite their established association with factors such as sun exposure in cutaneous melanomas,[3] gallstones in gallbladder cancer,[4] smoking in pancreatic cancer[5] and smoking and alcohol drinking in esophageal cancer.[6] Similarly, of 9 deterministic cancers, the relationship between hepatitis C and hepatocellular cancers is known since 1990s,[7] or about the causative association of human papilloma virus 16 in head and neck cancers.[8] Tomasetti and Vogelstein's analysis also assume that stem cell divisions are fixed within a specific tissue — with no racial/cultural difference.[2] Let's face it, if stem cell divisions are highly organ specific with no impact of other factors, then how do we explain the extremely large country based incidence of the “bad luck” cancers, viz. world-wide age-standardized incidence rates for cancers [Table 1].[9] Besides, setting aside all genetic evidences for intratumour heterogeneity,[10] if we accept randomness in cancer development any form of therapy (chemotherapy or radiotherapy) other than surgery (that would involve extirpation of the entire tumour), would be a worthless exercise. But the reality is that while surgery remains the mainstay of cancer treatment in, at least, solid organ cancers, not all patients present when their tumours are amenable to complete resection!
Table 1
Comparison of world-wide age standardized incidence rates for some cancers based on the IARC report
Shedding the nihilistic garb and looking at cancer management in a more positive light, there have been considerable improvements in cancer survival and not merely because these cancers have been detected at earlier stages but because of improvements in surgical technique and aggression, chemotherapy and radiation therapy.[11] And while it may be true that we may not have every answer to every cancer-related question, such as “why do cancers develop?” or “why don’t all patients with stage 1 cancers get cured by surgery alone” or “why is that some patients with stage 4 cancers live to 5 years while the majority do not make it past the 2nd year from diagnosis,” we do know that well done surgery adhering to oncologic principles and providing adjuvant therapy (chemo-and/or radio-therapy), when indicated, certainly do help to provide good outcomes. As for the cancer researcher, just because we do not have the answers today, certainly does not mean that the answers do not exist.
Most importantly, as an oncologist, one cannot forget that for the cancer patients that clinicians have to treat in the clinic every single day they are the only hope that they have on earth. And while they may not be able to answer every question or cure each and every patient, until they can, they must certainly offer the patients compassion and care that will soften the blow of cancer and help make the long arduous journey of a cancer patient a less unpleasant one. Maybe, just maybe, the words of Albert Einstein, “If you can’t explain it simply, you don’t understand it well enough” need to be recalled. In other words, we need to look more deeply at the 22 replicative cancers and all other not included in the analysis of Tomasetti and Vogelstein as the cause is lying out there waiting to be discovered!
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.


 PDF
PDF  Views
Views  Share
Share

