The masquerading splenic lesion
CC BY-NC-ND 4.0 · Indian J Med Paediatr Oncol 2016; 37(04): 311-313
DOI: DOI: 10.4103/0971-5851.195728

Publication History
Article published online:
12 July 2021
© 2016. Indian Society of Medical and Paediatric Oncology. This is an open access article published by Thieme under the terms of the Creative Commons Attribution-NonDerivative-NonCommercial-License, permitting copying and reproduction so long as the original work is given appropriate credit. Contents may not be used for commercial purposes, or adapted, remixed, transformed or built upon. (https://creativecommons.org/licenses/by-nc-nd/4.0/.)
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India
Sir,
Differential diagnosis for isolated splenic lesion can be organized around their basic imaging appearance as either predominantly cystic or solid.[1] The differential diagnosis should include benign entities, such as hemangioma, lymphangioma, hamartoma, inflammatory pseudotumor, infarct, abscess, and metastatic disease.
A 65-year-old previously healthy postmenopausal woman was admitted with a high-grade intermittent fever, cough with scanty yellowish sputum, and left-sided pleuritic chest pain for 1 month. She denied a history of weight loss, had no sick contacts, and had no history of addictions. On examination, she had a high-grade fever of 102°F, no tachycardia, and normal blood pressure. She had stony dull percussion note, coarse crepitation with reduced vocal fremitus, and vocal resonance over the left infraaxillary, infrascapular, and interscapular areas, suggesting left-sided synpneumonic effusion. There was no significant lymphadenopathy clubbing or intercostal tenderness. Hemoglobin was 8.7 g/dl (microcytic and hypochromic), total leukocyte count was 7400/ml with normal differential, platelet count of 280,000/µL, erythrocyte sedimentation rate was 84 mm in 1 h, and C-reactive protein was high. Blood chemistries were normal. Chest X-ray showed left lower zone homogeneous opacity without any air bronchogram [Figure 1a]. HIV, hepatitis B, and hepatitis C serologies were negative. Pleural fluid analysis was exudative with neutrophilic predominant pleocytosis. Pleural fluid Gram-stain, acid-fast Bacilli, and culture were negative. Sputum and blood cultures showed a heavy growth of Pseudomonas aeruginosa. A diagnosis of pseudomonal synpneumonic effusion with bacteremia was made, and she was started on piperacillin-tazobactam and ciprofloxacin. In spite of adequate antibiotic therapy based on culture reports for 4 weeks, fever, respiratory symptoms, signs, and chest X-ray findings persisted. Contrast-enhanced computed tomography (CT) of the chest showed moderate left pleural effusion, consolidation, and a focal heterogeneous area in the spleen with contrast enhancement (6.1 cm × 3.6 cm) [Figure 2a]. The splenic lesion was considered secondary to pseudomonas bacteremia after ruling out infective endocarditis by transthoracic echocardiogram. CT thorax and pleural fluid analysis were not suggestive of an underlying malignancy. Meanwhile, the mycobacterial cultures (BACTEC Mycobacteria Growth Indicator Tube 960) showed the growth of mycobacterium tuberculosis (TB). She was started on antitubercular drugs.
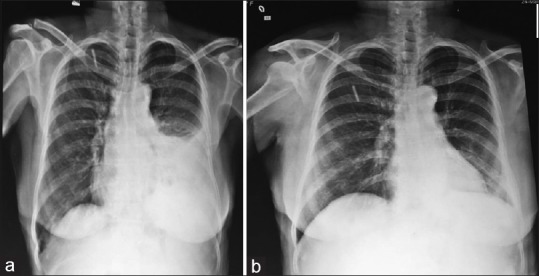
| Fig. 1(a) Initial chest X-ray showing left lower zone homogeneous opacity. (b) Repeat chest X-ray showing improvement
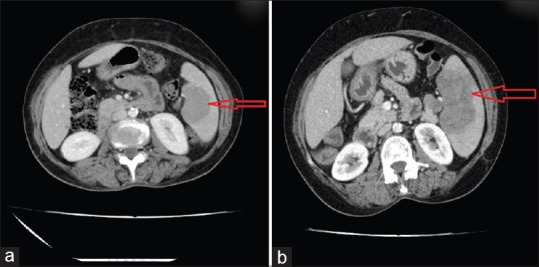
| Fig. 2(a) Initial contrast-enhanced computed tomography showing a focal heterogeneous area in the spleen with contrast enhancement (6.1 cm × 3.6 cm). (b) Repeat contrast-enhanced computed tomography of the abdomen showing increase in the heterogeneous area in the spleen measuring 8.2 cm × 6.4 cm with contrast enhancement
Two months after starting the antitubercular drugs, she was afebrile, respiratory symptoms and signs reduced, and chest X-ray showed mild left-sided pleural effusion [Figure 1b]. She was re-admitted 3 months after starting the antitubercular drugs with jaundice. She noticed yellowish discoloration of the sclera for 1 week, but did not have a history of fever, vomiting, itching, or clay-colored stools. A repeat physical examination demonstrated icterus and mild splenomegaly, which was firm and nontender. The liver function tests showed direct hyperbilirubinemia, moderately elevated transaminases with normal alkaline phosphatase and albumin. The prothrombin time and rest of the blood tests were normal. Hepatitis A, B, C, and E serologies were negative. Antinuclear antibody was negative. A repeat abdominal ultrasonogram showed normal liver and splenomegaly with an increase in size (8.2 cm × 6.4 cm) of the focal lesion in the spleen. Contrast-enhanced CT of the abdomen showed heterogeneous area in the spleen measuring 8.2 cm × 6.4 cm with contrast enhancement and perisplenic and perigastric nodes [Figure 2b].
The differential diagnosis for jaundice in our patient was infective, autoimmune, or drug induced. Antitubercular drugs were the likely culprit since the infective and autoimmune causes were ruled out by appropriate tests. The increase in the size of the splenic lesion was alarming since the patient showed clinical and radiological improvement after starting antitubercular drugs. Our patient had an accidentally detected splenic lesion on imaging which was attributed to the pseudomonal and mycobacterial infection initially.
Since there was a significant increase in the size of the splenic lesion on imaging, a tru-cut biopsy was performed which revealed large lymphoid cells arranged as sheets with pleomorphic vesicular nucleus, prominent nucleoli, and moderate cytoplasm [Figure 3a], and the cells showed confluent positivity for CD 20 [Figure 3b], weak positivity for Bcl-2 [Figure 3c], and Ki-67 positivity [Figure 3d]. The antitubercular drugs (isoniazid and rifampicin) were discontinued, and the patient was started on ethambutol and levofloxacin. Her liver function tests improved. Splenectomy followed by cyclophosphamide, adriamycin, vincristine, and prednisone chemotherapy was administered.
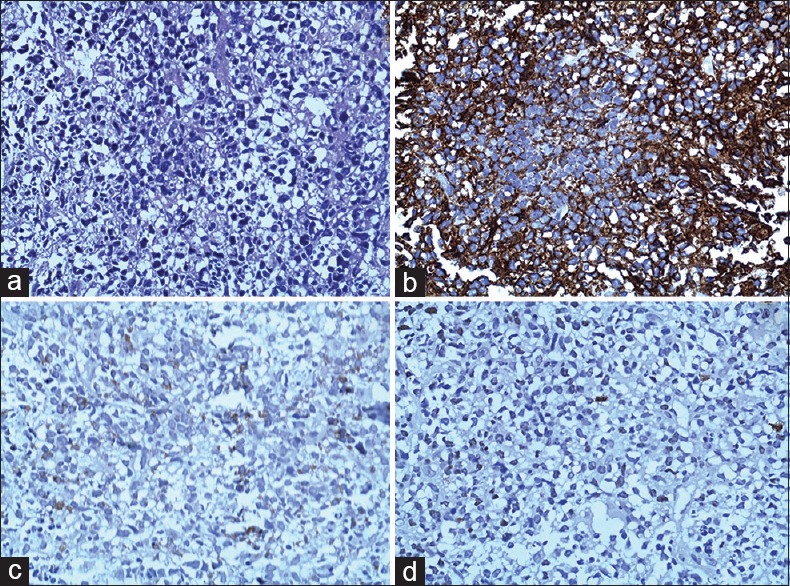
| Fig. 3 Splenic tru-cut biopsy showing large lymphoid cells arranged as sheets with pleomorphic vesicular nucleus, prominent nucleoli, and moderate cytoplasm (a), and the cells showed confluent positivity for CD 20 (b), weak positivity for Bcl-2 (c), and Ki-67 positivity (d), suggesting diffuse large B-cell lymphoma
Splenic pyogenous abscesses are caused by hematogenous spread of bacteria (Staphylococcus, Streptococcus, Escherichia coli, or Salmonella), penetrating trauma, prior splenic infarction, or contiguous infection.[2] Pyogenic abscesses are usually unilocular, but can be multifocal.[3] On contrast enhancement, pyogenic abscesses typically exhibit a “rim enhancement” of the outside-facing portion of the abscesses’ wall. Splenic abscess due to P. aeruginosa[4] and TB[5] was reported previously. The presence of pseudomonal bacteremia and pulmonary TB in our patient was considered as the cause of splenic lesion, even though the imaging features were not classical for an abscess. TB and lymphoma can be causatively related through the well-established lymphoma-related immunosuppression.[6] On the other direction, tuberculous infection causes direct DNA damage and apoptosis inhibition, which increase the mutagenesis of progeny cells, combined with angiogenesis favoring tumorigenesis.[7] P. aeruginosa bacteremia is a serious condition in patients with malignancy, and 50% of the infections are community-acquired.[8] Our patient had two infections, possibly secondary to lymphoma-related immunosuppression. Both infections (pseudomonas and tuberculous) can be associated with metastatic splenic lesions. Hence, the actual cause (B-cell lymphoma) was not revealed until the size of the lesion increased.
Primary splenic lymphoma is rare and occurs in approximately 1–2% of all lymphomas at presentation, most commonly diffuse large B-cell lymphoma. Primary splenic lymphoma is defined as lymphomatous involvement of the spleen with or without splenic hilar lymphadenopathy. Radiological and clinical appearances can mimic other splenic lesions including abscess that may delay the proper diagnosis and management. Lesions are of lower attenuation than the adjacent normal splenic parenchyma on unenhanced CT and demonstrate little or no enhancement following contrast.[9] Majority of the splenic lesions represent benign lesions that require no further workup. However, certain imaging characteristics such as ill-defined lesion borders, presence of solid, contrast-enhancing components, and increased attenuation of the lesion may help identify a potentially relevant disease. Splenic lesions detected on imaging have diverse causes, both benign and malignant, which should be evaluated carefully for proper diagnosis and management.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES

| Fig. 1(a) Initial chest X-ray showing left lower zone homogeneous opacity. (b) Repeat chest X-ray showing improvement

| Fig. 2(a) Initial contrast-enhanced computed tomography showing a focal heterogeneous area in the spleen with contrast enhancement (6.1 cm × 3.6 cm). (b) Repeat contrast-enhanced computed tomography of the abdomen showing increase in the heterogeneous area in the spleen measuring 8.2 cm × 6.4 cm with contrast enhancement

| Fig. 3 Splenic tru-cut biopsy showing large lymphoid cells arranged as sheets with pleomorphic vesicular nucleus, prominent nucleoli, and moderate cytoplasm (a), and the cells showed confluent positivity for CD 20 (b), weak positivity for Bcl-2 (c), and Ki-67 positivity (d), suggesting diffuse large B-cell lymphoma


 PDF
PDF  Views
Views  Share
Share

