The seroprevalence of Kaposi?s sarcoma associated herpes virus and human herpes virus-6 in pediatric patients with cancer and healthy children in a Turkish pediatric oncology center
CC BY-NC-ND 4.0 · Indian J Med Paediatr Oncol 2014; 35(03): 221-225
DOI: DOI: 10.4103/0971-5851.142039
Abstract
Background: Many studies have tried to be establish a pathogenic role for human herpesvirus-6 and -8 (HHV-6, HHV-8) in malignant diseases, but whether these viruses plays a role in these pathologies remains unclear. HHV-6 and HHV-8 seropositivity were shown in a healthy population. There is no published data in Turkey about seroprevalence of these viruses. We aimed to determine the seroprevalence of HHV-6 and HHV-8 in pediatric cancer patients and to compare with healthy Turkish children′s viral seroprevalence. Patients and Methods: Ninety-three pediatric cancer patients and 43 age-matched healthy children were included in the study. All sera were screened for antibodies to HHV-6 and HHV-8 by ELISA. Results: HHV-8 immunoglobulin G (IgG) was positive in 3.3% of lymphoma patients, in 4.8% of acute lymphoblastic leukemia (ALL) patients, in 4.8% of retinoblastoma patients and in 7% of healthy children. There was no significant difference in HHV-8 seroprevelance between these groups. HHV-6 seroprevalence was 81% in ALL patients, 70% in lymphoma group, 81% in retinoblastoma patients and 69.8% in healthy children. Although there was no significant difference in HHV-6 prevalence between healthy children and pediatric cancer patients, HHV-6 seropositivity tended to be higher in retinoblastoma patients under age of 4 years (odds ratio: 2.925). Conclusion: HHV-6 seroprevalence was higher than HHV-8 seropositivity in our study. Viral studies related HHV-6 seroprevelance in retinoblastoma patients would be useful to clarify if there is any etiological association between HHV-6 and retinoblastoma.
Publication History
Article published online:
19 July 2021
© 2014. Indian Society of Medical and Paediatric Oncology. This is an open access article published by Thieme under the terms of the Creative Commons Attribution-NonDerivative-NonCommercial-License, permitting copying and reproduction so long as the original work is given appropriate credit. Contents may not be used for commercial purposes, or adapted, remixed, transformed or built upon. (https://creativecommons.org/licenses/by-nc-nd/4.0/.)
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India
Abstract
Background:
Many studies have tried to be establish a pathogenic role for human herpesvirus-6 and -8 (HHV-6, HHV-8) in malignant diseases, but whether these viruses plays a role in these pathologies remains unclear. HHV-6 and HHV-8 seropositivity were shown in a healthy population. There is no published data in Turkey about seroprevalence of these viruses. We aimed to determine the seroprevalence of HHV-6 and HHV-8 in pediatric cancer patients and to compare with healthy Turkish children's viral seroprevalence.
Patients and Methods:
Ninety-three pediatric cancer patients and 43 age-matched healthy children were included in the study. All sera were screened for antibodies to HHV-6 and HHV-8 by ELISA.
Results:
HHV-8 immunoglobulin G (IgG) was positive in 3.3% of lymphoma patients, in 4.8% of acute lymphoblastic leukemia (ALL) patients, in 4.8% of retinoblastoma patients and in 7% of healthy children. There was no significant difference in HHV-8 seroprevelance between these groups. HHV-6 seroprevalence was 81% in ALL patients, 70% in lymphoma group, 81% in retinoblastoma patients and 69.8% in healthy children. Although there was no significant difference in HHV-6 prevalence between healthy children and pediatric cancer patients, HHV-6 seropositivity tended to be higher in retinoblastoma patients under age of 4 years (odds ratio: 2.925).
Conclusion:
HHV-6 seroprevalence was higher than HHV-8 seropositivity in our study. Viral studies related HHV-6 seroprevelance in retinoblastoma patients would be useful to clarify if there is any etiological association between HHV-6 and retinoblastoma.
INTRODUCTION
Human herpesvirus-8 (HHV-8), also defined Kaposi's sarcoma (KS)-associated herpes virus, which was identified by Chang et al. in 1994, is a novel human oncovirus classified as a gamma-herpesvirus.[1] HHV-8 is the causative agent of KS, but it has also been associated with different hematologic malignancies, including primary effusion lymphoma, multicentric Castelman's disease (MCD), MCD-related immunoblastic/plasmablastic lymphoma and various atypical lymphoproliferative disorders.[2,3,4,5,6]
Human herpesvirus-8 is unique among herpesviruses because its prevalence in the general population is low and because it possesses the richest weaponry of viral oncogenes and tumor-promoting factors ever described. Positivity in the seroprevalence studies of HHV-8 has found <4 href="https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4202619/#ref7" rid="ref7" class=" bibr popnode tag_hotlink tag_tooltip" id="__tag_409045674" role="button" aria-expanded="false" aria-haspopup="true" xss=removed>7]
Human herpesvirus-6 which was first isolated from immunocompromised patients with lymphoproliferative disorders[8], has been shown to be tropic in vitro for cells of the immune system, namely CD4+ T cells, B cells, natural killer cells and monocytes-macrophages; it is also infectious, although at a lower level, for glial cells and megakaryocytes.[9,10] Until date, huge numbers of investigations have examined the roles of HHV-6 in the development of hematological malignancies as an oncogenic agent.[11,12,13,14,15,16,17,18,19]
Human herpesvirus-6 is ubiquitous in the human adult population throughout the world, with seroconversion occurring early in life.[20,21]
We aimed to determine the seroprevalence of HHV-8 and HHV-6 in pediatric cancer patients at diagnosis as a risk factor and to compare with healthy Turkish children's HHV-8 and HHV-6 seroprevalence. In addition, as there is no published data in Turkey about seroprevalence of these viruses in children, we aimed to have knowledge about seroprevalence data in Turkey as a Mediterranean country.
PATIENTS AND METHODS
The study was performed on 93 newly diagnosed pediatric cancer patients with an age range of 3 months to 18 years. Thirty of patients were lymphoma (non-Hodgkin's lymphoma [NHL]: 22, HL: 8), 21 of patients were acute lymphoblastic leukemia (ALL) and 42 of patients were retinoblastoma. All patients presented to the Ankara University Medicine School Department of Pediatric Oncology, and all of them were diagnosed according to standard methods for their diseases.
Forty-three age-matched healthy children admitted to pediatrics, and well-baby clinics were included as a control group in the study.
All sera were separated from clotted whole blood by centrifugation and frozen at −20°C until analyzed. Testing for the HHV-8 and HHV-6 antibodies was performed by ELISA.
Statistical analysis was done using the Chi-square test for comparing independent qualitative data and logistic regression test to compare the patient's groups and the control group by “SPSS 11.5 for Windows” (Chicago inc. Licence code: 30001359390) statistical programme.
RESULTS
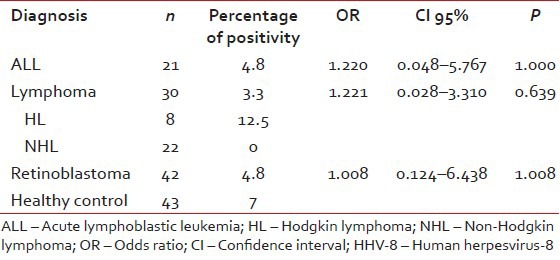
Human herpesvirus-8 immunoglobulin G (IgG) was positive in 3.3% of lymphoma patients (12.5% in HL, all of the NHL patients were negative), in 4.8% of ALL patients and in 4.8% of retinoblastoma patients. The prevalence of antibodies against to HHV-8 in healthy Turkish children was 7%. There was no significant difference in HHV-8 antibody prevalence between healthy children and pediatric cancer patients [Table 1].
Table 1
The prevelance of antibodies against to HHV-8
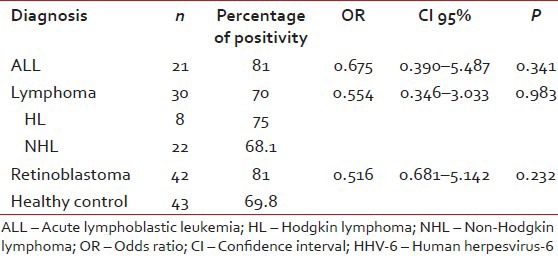
| Human herpesvirus-6 seroprevalence was 81% in ALL patients, 70% in lymphoma group (75% in HL, 40% in NHL patients) and 81% in retinoblastoma patients. In the healthy Turkish children group, rate of seropositivity to HHV-6 was 69.8%. Although HHV-6 seroprevelance was higher in ALL and retinoblastoma patients than the control group, there was no significant difference in HHV-6 antibody prevalence between healthy children and pediatric cancer patients [Table 2].
Table 2
The prevelance of antibodies against to HHV-6
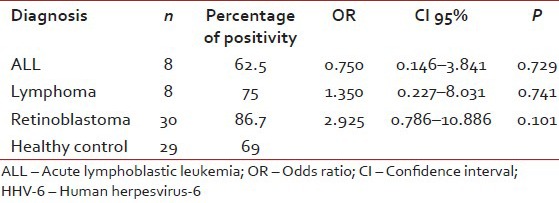
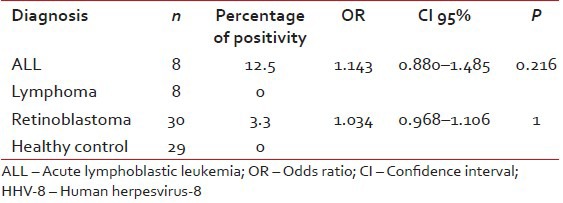
| Although there was no significance difference between patients and control groups for HHV-6 and HHV-8 seropositivities, healthy children and patients who under age of 4 years were compared in term of HHV-6 and HHV-8 seropositivities, since most retinoblastoma patients were under age of 4 years [Tables
[Tables33 and and44].Table 3
The prevelance of antibodies against to HHV-6 under age of 4 years
|
Table 4
The prevelance of antibodies against to HHV-8 under age of 4 years
|
DISCUSSION
The past two decades have seen significant advances describing the molecular pathways involved in HHV-8-induced malignancies. There appears to be an intricate interplay between the host immune system and the virus, which results in tumorigenesis with evasion of immune surveillance.[22,23,24,25,26,27]
In our study, HHV-8 IgG was positive in 3.3% of lymphoma patients (12.5% in HL, all of the NHL patients were negative), in 4.8% of ALL patients and 4.8% of retinoblastoma patients.
The seroprevalence of HHV-8 has been found to vary between studies, depending on the type of assay employed and the countries where the investigations were carried out. While Lennette et al. found that <4 href="https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4202619/#ref7" rid="ref7" class=" bibr popnode tag_hotlink tag_tooltip" id="__tag_409045705" role="button" aria-expanded="false" aria-haspopup="true" xss=removed>7], Cattani et al. showed that the seroprevalence of HHV-8 was 9.7% in children under age 15 in Mediterranean regions by immunofluorescence assay.[28]
The prevalence of antibodies to HHV-8 in healthy Turkish children was 7%. There was no significant difference in HHV-8 antibody prevelance between healthy children and pediatric cancer patients. Intrestingly, in the malignant lymphoma group, in terms of HHV-8, all of the HL patients showed seropositivity, whereas there was no seropositivity in NHL patients. However, statistical analysis was not feasible to show the difference between these two groups probably due to low number of the patients.
Human herpesvirus-6 is a ubiquitous HHV in the human adult population throughout the world, with seroconversion occurring early in life.[20,21] Like all other HHVs, once acquired, remain latent in the body, principally T lymphocytes, throughout life and reactivation occurs particularly in the case of immune suppression.[11,29]
Pathogenetic roles for HHV-6 in lymphoproliferative diseases have been of continued interest. Many molecular studies have tried to establish a pathogenic role for HHV-6 in lymphoid malignancies.[11,12,13,14,15,16,17,18,19] However, whether HHV-6 plays a role in these pathologies remains unclear, as positive polymerase chain reaction (PCR) results for HHV-6 in those studies may reflect latent infection or reactivation rather than the presence of HHV-6 in neoplastic cells.[12,15,16,17] A role for HHV-6 infection in hemodialysis (HD) was first suggested by serological studies showing that anti-HHV-6 antibody titers were higher in HD than in normal blood donors.[12] Since then, several studies aimed at identifying the HHV-6 genome in pathologic specimens using PCR have been reported in HD.[12,13,14,15,16,18] The HHV-6 genome was detected in 22.2-62.1% of cases of NHL by PCR.[12,13] In our study, we found that HHV-6 seroprevalence was 70% in lymphoma group (75% in HL, 68.1% in NHL patients).
The role of HHV-6 in acute leukemia, particulary childhood ALL, has been a matter of continuous interest, but remains controversial. Salonen et al. found high levels of HHV-6 antibodies in children with ALL compared with normal subjects.[19] By contrast, sequential study showed no significant differences in antibody titers between 50 patients with ALL and 50 sex-age matched blood donors.[30] HHV-6 sequences were first detected by PCR and in situ hybridization in the bone marrow cells of the majority of children with T-ALL[31], but a subsequent study showed that the presence of HHV-6 DNA is no more frequent in patients with ALL than in normal subjects.[32] Seror et al. recently analyzed HHV-6 DNA copy number by real-time PCR in bone marrow and peripheral blood from 36 children with ALL at diagnosis and during complete remission. They found lower viral load at diagnosis than in remission samples.[33] In our study, we found that HHV-6 seroprevalence was 81% in ALL patients.
There was no publication in the literature on HHV-6 seropositivity in patients with retinoblastoma, although HHV-6A-and HHV-6B-induced alterations in E2F1-Rb interactions, including protein levels, localization, phosphorylation, and the expression of exemplary target genes has been described.[34] E2F1 and its main repressor, Rb, serve as major regulators for the transcription of genes that function in cell cycle progression, viability, and apoptosis. The studies revealed significant alterations in the E2F1/Rb pathways at different points of viral infection. Especially, arrest at the G2 phase of cell cycle profiles of the virus-infected cells might have resulted from both p53 and the presence of complexes of E2F1/Rb early postinfection upon Rb dephosphorylation. Cell cycle arrest at early points in the infection may be advantageous for virus replication in dividing lymphocytes and arresting cell replication might enable the formation of more efficient viral spread. Eventually, the infection might lead to apoptosis and/or necrosis. Overall, the data presented in mentioned study shed new light on HHV-6 interaction with the infected cells, suggesting different manipulations of key factors relevant to cell proliferation and death.[34] We showed that HHV-6 seropositivity was 81% in 42 retinoblastoma patients.
In the healthy Turkish children group, rate of seropositivity to HHV-6 was 69.8%. Although HHV-6 seroprevelance was higher in ALL and retinoblastoma patients than the control group, there was no significant difference in HHV-6 antibody prevalence between healthy children and pediatric cancer patients. However, because of high-frequency retinoblastoma in patients under age of 4 years, we compared to healthy population and retinoblastoma patients who were under age of 4 years for HHV-6 seropositivity. Odds ratio was almost 3 times high (2.925) for retinoblastoma patients, although there were no statistical differences between two groups, probably related to the insufficient number of patients in the groups.
CONCLUSION
Human herpesvirus-6 seroprevalence rates were found to be higher than HHV-8 seropositivity rates in our patients and healthy group. In addition, high frequency of HHV-6 seropositivity was seen in retinoblastoma patients compared to healthy children under 4 years of age, although this was not statistically significant. According to the preliminary result of our serologic study, more HHV-6 DNA studies as a causative viral agent in retinoblastoma patients may help to reflect real association with retinoblastoma and HHV-6. However, the difficulties in differentiating infectious conditions from the malignancy are obvious. Proper studies at the onset of symptoms are extremely important for all patients suspected of having a malignant process. Many factors underline the need to develop new approaches for the study of malignant processes.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
References
- Chang Y, Cesarman E, Pessin MS, Lee F, Culpepper J, Knowles DM, et al. Identification of herpesvirus-like DNA sequences in AIDS-associated Kaposi′s sarcoma. Science 1994;266:1865-9.
- Cesarman E, Chang Y, Moore PS, Said JW, Knowles DM. Kaposi′s sarcoma-associated herpesvirus-like DNA sequences in AIDS-related body-cavity-based lymphomas. N Engl J Med 1995;332:1186-91.
- Soulier J, Grollet L, Oksenhendler E, Cacoub P, Cazals-Hatem D, Babinet P, et al. Kaposi′s sarcoma-associated herpesvirus-like DNA sequences in multicentric Castleman′s disease. Blood 1995;86:1276-80.
- Dupin N, Diss TL, Kellam P, Tulliez M, Du MQ, Sicard D, et al. HHV-8 is associated with a plasmablastic variant of Castleman disease that is linked to HHV-8-positive plasmablastic lymphoma. Blood 2000;95:1406-12.
- Du MQ, Diss TC, Liu H, Ye H, Hamoudi RA, Cabeçadas J, et al. KSHV- and EBV-associated germinotropic lymphoproliferative disorder. Blood 2002;100:3415-8.
- Ferry JA, Sohani AR, Longtine JA, Schwartz RA, Harris NL. HHV8-positive, EBV-positive Hodgkin lymphoma-like large B-cell lymphoma and HHV8-positive intravascular large B-cell lymphoma. Mod Pathol 2009;22:618-26.
- Lennette ET, Blackbourn DJ, Levy JA. Antibodies to human herpesvirus type 8 in the general population and in Kaposi′s sarcoma patients. Lancet 1996;348:858-61.
- Salahuddin SZ, Ablashi DV, Markham PD, Josephs SF, Sturzenegger S, Kaplan M, et al. Isolation of a new virus, HBLV, in patients with lymphoproliferative disorders. Science 1986;234:596-601.
- Kondo K, Kondo T, Shimada K, Amo K, Miyagawa H, Yamanishi K. Strong interaction between human herpesvirus 6 and peripheral blood monocytes/macrophages during acute infection. J Med Virol 2002;67:364-9.
- ;Kondo K, Kondo T, Okuno T, Takahashi M, Yamanishi K. Latent human herpesvirus 6 infection of human monocytes/macrophages. J Gen Virol 1991;72:1401-8.
- ;Ogata M. Human herpesvirus 6 in hematological malignancies. J Clin Exp Hematop 2009;49:57-67.
- Torelli G, Marasca R, Luppi M, Selleri L, Ferrari S, Narni F, et al. Human herpesvirus-6 in human lymphomas: identification of specific sequences in Hodgkin′s lymphomas by polymerase chain reaction. Blood 1991;77:2251-8.
- Sumiyoshi Y, Kikuchi M, Ohshima K, Takeshita M, Eizuru Y, Minamishima Y. Analysis of human herpes virus-6 genomes in lymphoid malignancy in Japan. J Clin Pathol 1993;46:1137-8.
- Lacroix A, Jaccard A, Rouzioux C, Piguet C, Petit B, Bordessoule D, et al. HHV-6 and EBV DNA quantitation in lymph nodes of 86 patients with Hodgkin′s lymphoma. J Med Virol 2007;79:1349-56.
- Trovato R, Di Lollo S, Calzolari A, Torelli G, Ceccherini-Nelli L. Detection of human herpesvirus-6 and Epstein-Barr virus genome in childhood Hodgkin′s disease. Pathologica 1994;86:500-3.
- Valente G, Secchiero P, Lusso P, Abete MC, Jemma C, Reato G, et al. Human herpesvirus 6 and Epstein-Barr virus in Hodgkin′s disease: a controlled study by polymerase chain reaction and in situ hybridization. Am J Pathol 1996;149:1501-10.
- Luppi M, Barozzi P, Garber R, Maiorana A, Bonacorsi G, Artusi T, et al. Expression of human herpesvirus-6 antigens in benign and malignant lymphoproliferative diseases. Am J Pathol 1998;153:815-23.
- Schmidt CA, Oettle H, Peng R, Binder T, Wilborn F, Huhn D, et al. Presence of human beta- and gamma-herpes virus DNA in Hodgkin′s disease. Leuk Res 2000;24:865-70.
- Salonen MJ, Siimes MA, Salonen EM, Vaheri A, Koskiniemi M. Antibody status to HHV-6 in children with leukaemia. Leukemia 2002;16:716-9.
- Yoshikawa T, Suga S, Asano Y, Yazaki T, Kodama H, Ozaki T. Distribution of antibodies to a causative agent of exanthem subitum (human herpesvirus-6) in healthy individuals. Pediatrics 1989;84:675-7.
- Zerr DM, Meier AS, Selke SS, Frenkel LM, Huang ML, Wald A, et al. A population-based study of primary human herpesvirus 6 infection. N Engl J Med 2005;352:768-76.
- Liang C, Lee JS, Jung JU. Immune evasion in Kaposi′s sarcoma-associated herpes virus associated oncogenesis. Semin Cancer Biol 2008;18:423-36.
- Wen KW, Damania B. Kaposi sarcoma-associated herpesvirus (KSHV): molecular biology and oncogenesis. Cancer Lett 2010;289:140-50.
- Sunil M, Reid E, Lechowicz MJ. Update on HHV-8-Associated Malignancies. Curr Infect Dis Rep 2010;12:147-54.
- Cathomas G. Kaposi′s sarcoma-associated herpesvirus (KSHV)/human herpesvirus 8 (HHV-8) as a tumour virus. Herpes 2003;10:72-7.
- Radkov SA, Kellam P, Boshoff C. The latent nuclear antigen of Kaposi sarcoma-associated herpesvirus targets the retinoblastoma-E2F pathway and with the oncogene Hras transforms primary rat cells. Nat Med 2000;6:1121-7.
- Sullivan RJ, Pantanowitz L, Casper C, Stebbing J, Dezube BJ. HIV/AIDS: epidemiology, pathophysiology, and treatment of Kaposi sarcoma-associated herpesvirus disease: Kaposi sarcoma, primary effusion lymphoma, and multicentric Castleman disease. Clin Infect Dis 2008;47:1209-15.
- Cattani P, Cerimele F, Porta D, Graffeo R, Ranno S, Marchetti S, et al. Age-specific seroprevalence of Human Herpesvirus 8 in Mediterranean regions. Clin Microbiol Infect 2003;9:274-9.
- Zerr DM. Human herpesvirus 6: a clinical update. Herpes 2006;13:20-4.
- Levine PH, Ablashi DV, Saxinger WC, Connelly RR. Antibodies to human herpes virus-6 in patients with acute lymphocytic leukemia. Leukemia 1992;6:1229-31.
- Luka J, Pirruccello SJ, Kersey JH. HHV-6 genome in T-cell acute lymphoblastic leukaemia. Lancet 1991;338:1277-8.
- Barozzi P, Luppi M, Marasca R, Trovato R, Ceccherini-Nelli L, Torelli G. Human herpesvirus-6 genome in acute lymphoblastic leukemia: evidence against an etiologic relationship. Acta Haematol 1995;94:169-72.
- Seror E, Coquerel B, Gautheret-Dejean A, Ballerini P, Landman-Parker J, Leverger G, et al. Quantitation of Human herpes virus 6 genome in children with acute lymphoblastic leukemia. J Med Virol 2008;80:689-93.
- Mlechkovich G, Frenkel N. Human herpesvirus 6A (HHV-6A) and HHV-6B alter E2F1/Rb pathways and E2F1 localization and cause cell cycle arrest in infected T cells. J Virol 2007;81:13499-508.
References
- Chang Y, Cesarman E, Pessin MS, Lee F, Culpepper J, Knowles DM, et al. Identification of herpesvirus-like DNA sequences in AIDS-associated Kaposi′s sarcoma. Science 1994;266:1865-9.
- Cesarman E, Chang Y, Moore PS, Said JW, Knowles DM. Kaposi′s sarcoma-associated herpesvirus-like DNA sequences in AIDS-related body-cavity-based lymphomas. N Engl J Med 1995;332:1186-91.
- Soulier J, Grollet L, Oksenhendler E, Cacoub P, Cazals-Hatem D, Babinet P, et al. Kaposi′s sarcoma-associated herpesvirus-like DNA sequences in multicentric Castleman′s disease. Blood 1995;86:1276-80.
- Dupin N, Diss TL, Kellam P, Tulliez M, Du MQ, Sicard D, et al. HHV-8 is associated with a plasmablastic variant of Castleman disease that is linked to HHV-8-positive plasmablastic lymphoma. Blood 2000;95:1406-12.
- Du MQ, Diss TC, Liu H, Ye H, Hamoudi RA, Cabeçadas J, et al. KSHV- and EBV-associated germinotropic lymphoproliferative disorder. Blood 2002;100:3415-8.
- Ferry JA, Sohani AR, Longtine JA, Schwartz RA, Harris NL. HHV8-positive, EBV-positive Hodgkin lymphoma-like large B-cell lymphoma and HHV8-positive intravascular large B-cell lymphoma. Mod Pathol 2009;22:618-26.
- Lennette ET, Blackbourn DJ, Levy JA. Antibodies to human herpesvirus type 8 in the general population and in Kaposi′s sarcoma patients. Lancet 1996;348:858-61.
- Salahuddin SZ, Ablashi DV, Markham PD, Josephs SF, Sturzenegger S, Kaplan M, et al. Isolation of a new virus, HBLV, in patients with lymphoproliferative disorders. Science 1986;234:596-601.
- Kondo K, Kondo T, Shimada K, Amo K, Miyagawa H, Yamanishi K. Strong interaction between human herpesvirus 6 and peripheral blood monocytes/macrophages during acute infection. J Med Virol 2002;67:364-9.
- ;Kondo K, Kondo T, Okuno T, Takahashi M, Yamanishi K. Latent human herpesvirus 6 infection of human monocytes/macrophages. J Gen Virol 1991;72:1401-8.
- ;Ogata M. Human herpesvirus 6 in hematological malignancies. J Clin Exp Hematop 2009;49:57-67.
- Torelli G, Marasca R, Luppi M, Selleri L, Ferrari S, Narni F, et al. Human herpesvirus-6 in human lymphomas: identification of specific sequences in Hodgkin′s lymphomas by polymerase chain reaction. Blood 1991;77:2251-8.
- Sumiyoshi Y, Kikuchi M, Ohshima K, Takeshita M, Eizuru Y, Minamishima Y. Analysis of human herpes virus-6 genomes in lymphoid malignancy in Japan. J Clin Pathol 1993;46:1137-8.
- Lacroix A, Jaccard A, Rouzioux C, Piguet C, Petit B, Bordessoule D, et al. HHV-6 and EBV DNA quantitation in lymph nodes of 86 patients with Hodgkin′s lymphoma. J Med Virol 2007;79:1349-56.
- Trovato R, Di Lollo S, Calzolari A, Torelli G, Ceccherini-Nelli L. Detection of human herpesvirus-6 and Epstein-Barr virus genome in childhood Hodgkin′s disease. Pathologica 1994;86:500-3.
- Valente G, Secchiero P, Lusso P, Abete MC, Jemma C, Reato G, et al. Human herpesvirus 6 and Epstein-Barr virus in Hodgkin′s disease: a controlled study by polymerase chain reaction and in situ hybridization. Am J Pathol 1996;149:1501-10.
- Luppi M, Barozzi P, Garber R, Maiorana A, Bonacorsi G, Artusi T, et al. Expression of human herpesvirus-6 antigens in benign and malignant lymphoproliferative diseases. Am J Pathol 1998;153:815-23.
- Schmidt CA, Oettle H, Peng R, Binder T, Wilborn F, Huhn D, et al. Presence of human beta- and gamma-herpes virus DNA in Hodgkin′s disease. Leuk Res 2000;24:865-70.
- Salonen MJ, Siimes MA, Salonen EM, Vaheri A, Koskiniemi M. Antibody status to HHV-6 in children with leukaemia. Leukemia 2002;16:716-9.
- Yoshikawa T, Suga S, Asano Y, Yazaki T, Kodama H, Ozaki T. Distribution of antibodies to a causative agent of exanthem subitum (human herpesvirus-6) in healthy individuals. Pediatrics 1989;84:675-7.
- Zerr DM, Meier AS, Selke SS, Frenkel LM, Huang ML, Wald A, et al. A population-based study of primary human herpesvirus 6 infection. N Engl J Med 2005;352:768-76.
- Liang C, Lee JS, Jung JU. Immune evasion in Kaposi′s sarcoma-associated herpes virus associated oncogenesis. Semin Cancer Biol 2008;18:423-36.
- Wen KW, Damania B. Kaposi sarcoma-associated herpesvirus (KSHV): molecular biology and oncogenesis. Cancer Lett 2010;289:140-50.
- Sunil M, Reid E, Lechowicz MJ. Update on HHV-8-Associated Malignancies. Curr Infect Dis Rep 2010;12:147-54.
- Cathomas G. Kaposi′s sarcoma-associated herpesvirus (KSHV)/human herpesvirus 8 (HHV-8) as a tumour virus. Herpes 2003;10:72-7.
- Radkov SA, Kellam P, Boshoff C. The latent nuclear antigen of Kaposi sarcoma-associated herpesvirus targets the retinoblastoma-E2F pathway and with the oncogene Hras transforms primary rat cells. Nat Med 2000;6:1121-7.
- Sullivan RJ, Pantanowitz L, Casper C, Stebbing J, Dezube BJ. HIV/AIDS: epidemiology, pathophysiology, and treatment of Kaposi sarcoma-associated herpesvirus disease: Kaposi sarcoma, primary effusion lymphoma, and multicentric Castleman disease. Clin Infect Dis 2008;47:1209-15.
- Cattani P, Cerimele F, Porta D, Graffeo R, Ranno S, Marchetti S, et al. Age-specific seroprevalence of Human Herpesvirus 8 in Mediterranean regions. Clin Microbiol Infect 2003;9:274-9.
- Zerr DM. Human herpesvirus 6: a clinical update. Herpes 2006;13:20-4.
- Levine PH, Ablashi DV, Saxinger WC, Connelly RR. Antibodies to human herpes virus-6 in patients with acute lymphocytic leukemia. Leukemia 1992;6:1229-31.
- Luka J, Pirruccello SJ, Kersey JH. HHV-6 genome in T-cell acute lymphoblastic leukaemia. Lancet 1991;338:1277-8.
- Barozzi P, Luppi M, Marasca R, Trovato R, Ceccherini-Nelli L, Torelli G. Human herpesvirus-6 genome in acute lymphoblastic leukemia: evidence against an etiologic relationship. Acta Haematol 1995;94:169-72.
- Seror E, Coquerel B, Gautheret-Dejean A, Ballerini P, Landman-Parker J, Leverger G, et al. Quantitation of Human herpes virus 6 genome in children with acute lymphoblastic leukemia. J Med Virol 2008;80:689-93.
- Mlechkovich G, Frenkel N. Human herpesvirus 6A (HHV-6A) and HHV-6B alter E2F1/Rb pathways and E2F1 localization and cause cell cycle arrest in infected T cells. J Virol 2007;81:13499-508.


 PDF
PDF  Views
Views  Share
Share

