Thermo Mammogram as a Tool to Assess Response to Neoadjuvant Chemotherapy in Breast Carcinoma
CC BY-NC-ND 4.0 ? Indian J Med Paediatr Oncol 2019; 40(S 01): S25-S32
DOI: DOI: 10.4103/ijmpo.ijmpo_144_17
Abstract
Introduction:?Response to neoadjuvant chemotherapy (NACT) is predicted by clinical examination alone in locally advanced breast carcinoma. This study uses thermo mammogram (TMG) to assess the response.?Aim and Objectives:?The aim is to study TMG changes during NACT in breast cancer and predict response to NACT in locally advanced carcinoma and to compare clinical response with TMG response/changes in any form. Patients and?Methods:?All patients with locally advanced breast cancer who had treated with NACT were included in this study. Baseline TMG picture was taken using illumina360? (digital robotic rotational thermography device for 360 degree view of each breast) system before chemotherapy. TMG was repeated before next cycle. All patients were also assessed clinically during and after each cycle of chemotherapy. To assess the potential of TMG in predicting tissue response to chemotherapy, the precool, postcool, and the temperature difference between precool and postcool before every cycle were analyzed.?Results:?A total of 19 patients were analyzed. Eight patients had complete clinical response, six patients had partial response, and five patients had static disease. Median of precool, temperature difference between precool and postcool for patients between no response and complete response did not show statistically significant difference. However, the median of postcool spot temperature showed statistically significant difference. Median of postcool temperature difference for patients between partial response and complete response showed statistically significant difference. The median of postcool spot temperature for patients with no response and partial response did not show statistically significant difference. Precool temperature difference for all the visits showed no statistically significant difference.?Conclusion:?This preliminary study suggests that the TMG has potential for monitoring NACT response in breast cancer patients. Postcool temperature measurement is an early indicator of response to NACT.
Publication History
Article published online:
24 May 2021
? 2019. Indian Society of Medical and Paediatric Oncology. This is an open access article published by Thieme under the terms of the Creative Commons Attribution-NonDerivative-NonCommercial-License, permitting copying and reproduction so long as the original work is given appropriate credit. Contents may not be used for commercial purposes, or adapted, remixed, transformed or built upon. (https://creativecommons.org/licenses/by-nc-nd/4.0/).
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India
Abstract
Introduction:?Response to neoadjuvant chemotherapy (NACT) is predicted by clinical examination alone in locally advanced breast carcinoma. This study uses thermo mammogram (TMG) to assess the response.?Aim and Objectives:?The aim is to study TMG changes during NACT in breast cancer and predict response to NACT in locally advanced carcinoma and to compare clinical response with TMG response/changes in any form. Patients and?Methods:?All patients with locally advanced breast cancer who had treated with NACT were included in this study. Baseline TMG picture was taken using illumina360? (digital robotic rotational thermography device for 360 degree view of each breast) system before chemotherapy. TMG was repeated before next cycle. All patients were also assessed clinically during and after each cycle of chemotherapy. To assess the potential of TMG in predicting tissue response to chemotherapy, the precool, postcool, and the temperature difference between precool and postcool before every cycle were analyzed.?Results:?A total of 19 patients were analyzed. Eight patients had complete clinical response, six patients had partial response, and five patients had static disease. Median of precool, temperature difference between precool and postcool for patients between no response and complete response did not show statistically significant difference. However, the median of postcool spot temperature showed statistically significant difference. Median of postcool temperature difference for patients between partial response and complete response showed statistically significant difference. The median of postcool spot temperature for patients with no response and partial response did not show statistically significant difference. Precool temperature difference for all the visits showed no statistically significant difference.?Conclusion:?This preliminary study suggests that the TMG has potential for monitoring NACT response in breast cancer patients. Postcool temperature measurement is an early indicator of response to NACT.
Introduction
Infrared imaging of the breast is commonly known as breast thermography, painless examination, and a noninvasive modality which mainly measures the temperature of the breasts and does not expose the subject to toxic ionizing radiation and tests a subtle changes of physiologic response of the breast.[1] [2] [3] [4] [5] [6] Thermographic imaging has already been clinically used as a screening and diagnosis tool to detect the breast cancer, where high blood flow and metabolic activity due to high vascularity of the tumor is warmer than the surrounding normal functioning tissue. Thermographic or thermo mammogram (TMG) imaging can examine both tumor growth and vasculature changes.[7] Recently, breast thermograms are widely applied for the precise detection of breast cancer worldwide.[8] [9] [10] [11] [12] [13] [14]
Most of the patients diagnosed with advanced breast cancer undergo preoperative neoadjuvant chemotherapy (NACT).[15] Patients with pathological complete response for the NACT are associated with longer disease-free survival.[16] [17] [18] Unfortunately, between 8% and 20% of breast cancer patients will not benefit from NACT and will be treated without clinical or pathologic response.[16] [19] Diagnostic methods to predict early response during therapy would help physicians to decide evidence-based changes on treatment strategies and potentially minimizing side effects and maximizing therapeutic outcome.
At present, most commonly used assessment of response to NACT is by clinical examination alone which is subjective in nature. Many other methods that are in practice to monitor the NACT response include ultrasonography, mammography and contrast-enhanced magnetic resonance imaging (MRI), magnetic resonance spectroscopy, and functional imaging technologies such as 18F-fluorodeoxyglucose positron emission tomography. Mammography is currently the most efficient imaging method for the detection of breast cancer with an accuracy of around 90% for screening. Although many treatment guidelines recommend mammography, the difficulty in assessing the tumor size is problematic when mammography is used for an application in chemotherapy response evaluation in chemotherapy response evaluation.[20] [21] [22] Even, some of the recent methods including functional imaging technologies require exogenous contrast agents that may be poorly tolerated by some patients and are performed at significant expense.[23] [24] Hence, practical limitations include preventing many of these methods from being applied in clinical practice for the use.[23]
This study aims to analyze the temperature variations for the response to NACT in locally advanced breast carcinoma using TMG by the following:
Whether thermography is useful to monitor the neoadjuvant therapy
Is there any possibility of using information obtained from thermography to predict the response to therapy?
What is the prediction model? Or proposed prediction model?
Patients and Methods
This is single-center, prospective cross-sectional study. The study included 19 consecutive locally advanced breast cancer patients who were undergoing NACT during the period from January 2016 to September 2016. Before the start of the study, signed informed consent was obtained from all patients, whose breasts were imaged by Illumina360? with minimum of 21-day interval up to minimum of three cycles of chemotherapy.
All patients were also assessed clinically and sonomammographically [Figure 1] before every chemotherapy cycle and imaging session. Data on grade, histologic subtype, size, and tumor response as per clinical assessment were recorded for all patients.
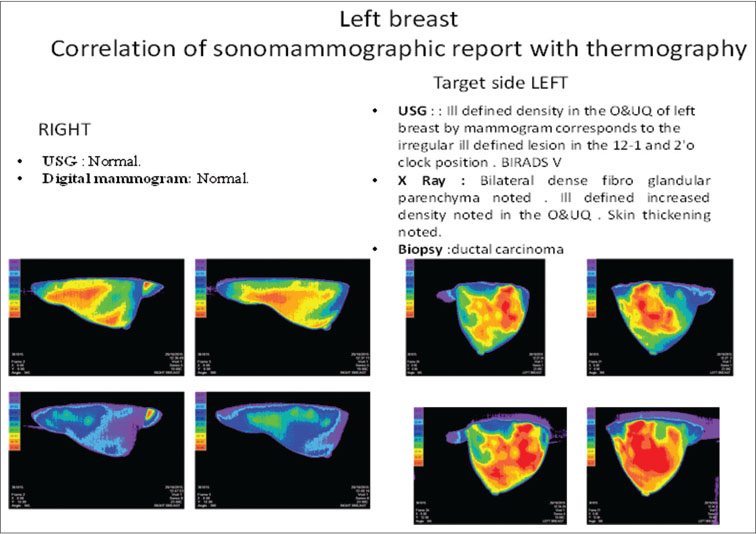
|?Figure. 1? Correlation of sonomammogram with thermo mammogram|
Patients were categorized as follows:
Complete response: Complete disappearance of all disease
Partial response: ?30% reduction in the sum of the longest diameter of target lesions
Stable disease: Change not meeting criteria for response or progression
Progression: ?20% increase in the sum of the longest diameter of target lesions
Stable disease and progressive disease are considered as no response to chemotherapy.
Procedure for thermography technique
Thermography images were taken using Illumina360?, a medical device for breast imaging without involving any radiation or contact with the patient breast. The subject lay prone on the imaging bed during the procedure with breast suspended through the opening in the top of the bed. Each breast was imaged individually. Infrared imaging began with a brief period of temperature stasis, after which a stream of cool air was circulated within the temperature conditioning chamber around the uncovered suspended breast. Multiple infrared images were taken one at every 15? angle by the infrared camera both before and after the cooling phase. After the first breast was imaged, the process was repeated for the contralateral breast. The entire session required approximately 15?20 min. Images were acquired in the affected side and the other side.
Statistical analysis
All baseline data (demographic and characteristics) and endpoint data are summarized with descriptive statistics ? median and interquartile range (25%?75%). Normality violations for each parameter were checked using the Shapiro?Wilk test. We categorized the final disease status as a categorical variable, that is, no response, partial response, or complete response. Nonparametric Mann?Whitney test (two-sided, 95% confidence) was used to calculate for statistical significant changes in thermography temperature in cycle 1, cycle 2, and cycle 3 between no response, partial response, and complete response. Statistical significance level of?P?< 0>
Results
Patients and tumor characteristics
The clinical characteristics of the patients and their tumors are summarized in [Table 1]. The median age of female patient was 50 years and seven patients were normally menstruating. Patients had a different combination of neoadjuvant treatment plans. Most of the patients had combined Adriamycin and paclitaxel-based chemotherapy. Of the 19 patients, three had tumor Grade 1, 12 patients had tumor Grade 2, and the rest, four patients had tumor Grade 3. Neoadjuvant treatment response is usually good for high-grade (2 or 3) tumors. However, in our study, only two patients had complete response. Estrogen/progesterone receptor (ER/PR) marker-positive patients responded very low for neoadjuvant treatment. As expected same in our study, among nine ER/PR-positive patients, seven patients were failed to get complete treatment responses. If HER2 biomarker is positive in histopathology, neoadjuvant treatment response is good. Even though seven patients had HER2-neu positive, only one patient had complete response.
|
Patient ID |
Age |
Menopause status (pre/post) |
Tumor size (at V1) |
Histology |
Grade |
ER/PR |
HER2 |
Neoadjuvant treatment |
Tumor size (at V3) |
|---|---|---|---|---|---|---|---|---|---|
|
Where the neoadjuvant treatment markings are as mentioned below: (A) ? Adriamycin; (C) ? Cyclophosphamide; (D) ? Docetaxel; (P) ? Paclitaxel. IDC ? Infiltrating ductal carcinoma; ILC ? Invasive lobular carcinoma; ER ? Estrogen receptors; PR ? Progesterone receptors |
|||||||||
|
Complete response |
|||||||||
|
65 |
51 |
Post |
5.8 cm ? 2.4 cm |
IDC |
1 |
-/- |
- |
(A) (P) |
Nil |
|
44 |
55 |
Post |
4 cm ? 3 cm |
IDC |
3 |
+/+ |
- |
(D) (P) |
Nil |
|
83 |
56 |
Post |
6 cm ? 5 cm |
IDC |
2 |
-/- |
+ |
(P)(A) |
Nil |
|
92 |
58 |
Post |
6 cm ? 5 cm |
IDC |
3 |
+/+ |
+ |
(A)(C) |
Nil |
|
108 |
40 |
Pre |
8 cm ? 6 cm |
IDC |
2 |
-/- |
+ |
(P)(A) |
Nil |
|
118 |
58 |
Post |
6 cm ? 5 cm |
IDC |
1 |
+/- |
+ |
(D) (A) (C) |
Nil |
|
136 |
48 |
Pre |
2.6 cm ? 1.8 cm |
IDC |
2 |
-/- |
- |
(A) (P) (C) |
Nil |
|
138 |
46 |
Post |
6 cm ? 4 cm |
IDC |
2 |
+/- |
- |
(A) (P) |
Nil |
|
Partial response |
|||||||||
|
6 |
33 |
Pre |
8 cm ? 5 cm |
IDC |
1 |
+ |
(A)(C) |
2 cm ? 2cm |
|
|
45 |
58 |
Post |
8 cm ? 7 cm |
IDC |
2 |
-/- |
- |
(A) (C) |
2 cm ? 2cm |
|
47 |
42 |
Pre |
Enlarged entire breast IDC |
2 |
+/+ |
- |
(A) (C) (P) |
Reduction noticed |
|
|
105 |
55 |
Post |
6 cm ? 4 cm |
IDC |
2 |
-/- |
+ |
(A)(P) |
3 cm ? 2 cm |
|
129 |
47 |
Pre |
8 cm ? 6 cm |
IDC |
2 |
-/- |
- |
(A) (C) |
3 cm ? 3cm |
|
142 |
57 |
Post |
7 cm ? 6cm |
IDC |
2 |
+/+ |
- |
(A) (P) |
5 cm ? 4cm |
|
No response |
|||||||||
|
25 |
48 |
Post |
8 cm ? 6 cm |
IDC |
3 |
- |
(A) (C) |
8 cm ? 6 cm |
|
|
67 |
56 |
Post |
5 cm ? 4 cm |
IDC |
2 |
-/- |
- |
(A) (P) |
6 cm ? 5 cm |
|
91 |
52 |
Post |
8 cm ? 6cm |
ILC |
2 |
-/- |
- |
(A) (C) |
8 cm ? 6 cm |
|
114 |
42 |
Pre |
15 cm ? 12 cm |
IDC |
3 |
-/- |
+ |
(D) (A) (C) |
12 cm ? 10 cm |
|
147 |
36 |
Pre |
8 cm ? 6 cm |
IDC |
2 |
- |
(A) (C) (P) |
5 cm ? 5 cm |
|
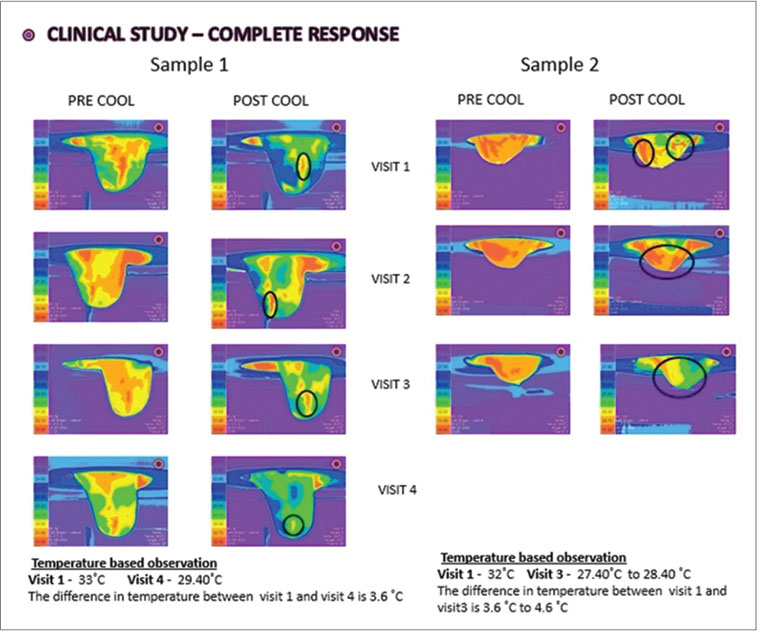
|?Figure. 2? Thermo mammographic images of complete response sample cases
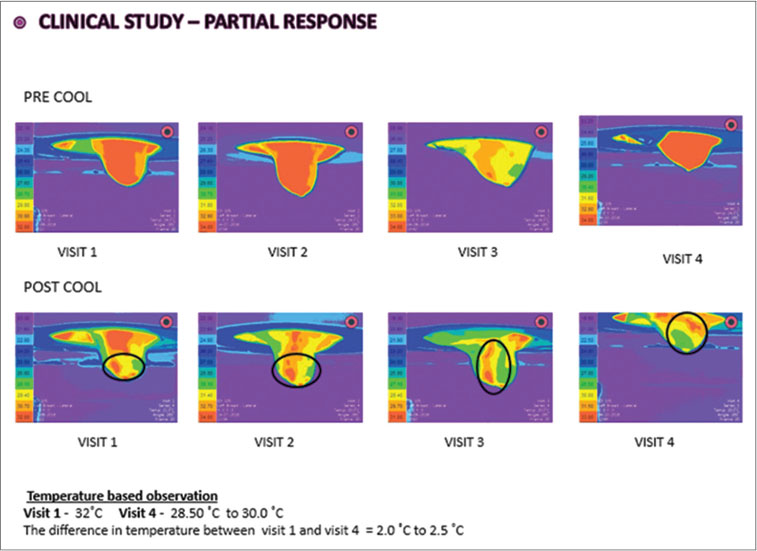
|?Figure. 3? Thermo mammographic images of partial response sample case
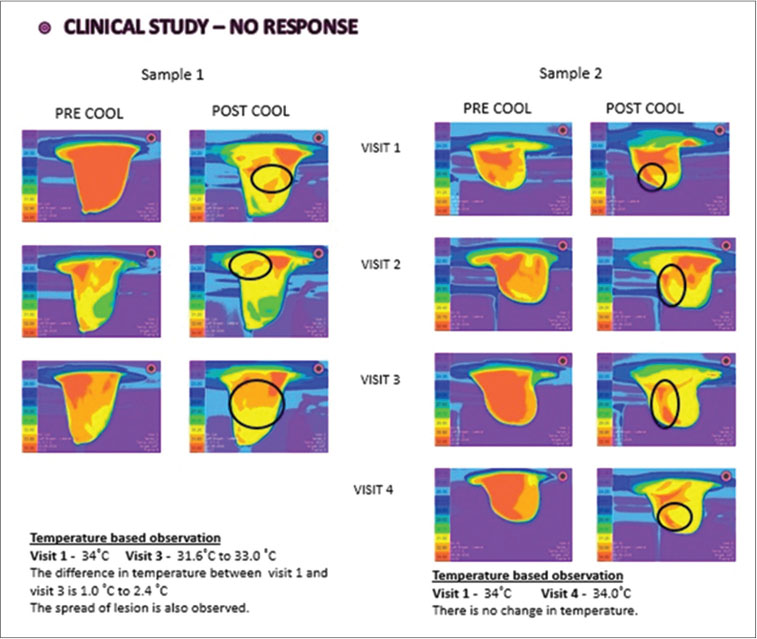
|?Figure. 4? Thermo mammographic images of no response sample cases
Thermo mammogram assessment of tumor response
Overall, 19 patients? thermography details were collected for three cycles of neoadjuvant treatment. To assess the potential of thermography in predicting early response, the precool, postcool, and temperature difference before every cycle were analyzed.
Median of precool, temperature difference for patients between no response and complete response did not show statistically significant difference. However, median of postspot temperature for patients in Visit 1 (34.0 vs. 31.5)?P?< 0 class="i" xss=removed>P?< 0 class="i" xss=removed>P?< 0 href="https://www.thieme-connect.com/products/ejournals/html/10.4103/ijmpo.ijmpo_144_17#TB_2" xss=removed>Table 2].
|
Median (25th and 75th percentile) |
P* |
|||||
|---|---|---|---|---|---|---|
|
No response |
Partial response |
Complete response |
No response versus complete response |
No response versus partial response |
Partial response versus complete response |
|
|
*Mann?Whitney test. NS: Not significant |
||||||
|
Visit 1 |
||||||
|
Precool spot temp |
35.0 (34.036.0) |
35.0 (33.0-35.0) |
32.5 (32.0-33.0) |
NS |
NS |
NS |
|
Postcool spot temp-no response |
34.0 (32.0-34.0) |
33.0 (32.0-34.0) |
31.5 (30.0-33.0) |
P<0> |
NS |
P<0> |
|
?Temp (precool, postcool) spot temp -no response |
2.0 (1-2.5) |
2.0 (0-2.0) |
1.5 (-1-3.75) |
NS |
NS |
NS |
|
Visit 2 |
||||||
|
Precool spot temp |
34.0 (32.5-36.0) |
34.0 (34.0-35.0) |
32.0 (32.0-33.75) |
NS |
NS |
NS |
|
Postcool spot temp |
33.0 (32.5-34.5) |
33.0 (32.0-34.0) |
30.0 (29.17-32.55) |
P<0> |
NS |
P<0> |
|
ATemp (precool, postcool) spot temp |
1.00 (-0.5-2.5) |
1.00 (0-3.0) |
2.00 (1.1-2.75) |
NS |
NS |
NS |
|
Visit 3 |
||||||
|
Precool spot temp |
35.00 (32.0-36.0) |
34.00 (34.0-34.0) |
33.00 (32.38-34.0) |
NS |
NS |
NS |
|
Postcool spot temp |
33.00 (30.5-35.0) |
32.00 (32.0-33.0) |
30.60 (28.80-31.0) |
P<0> |
NS |
P<0> |
|
ATemp (precool, postcool) spot temp |
1.00 (0-3.00) |
2.00 (1-3.00) |
2.95 (1.4-5.03) |
NS |
NS |
NS |
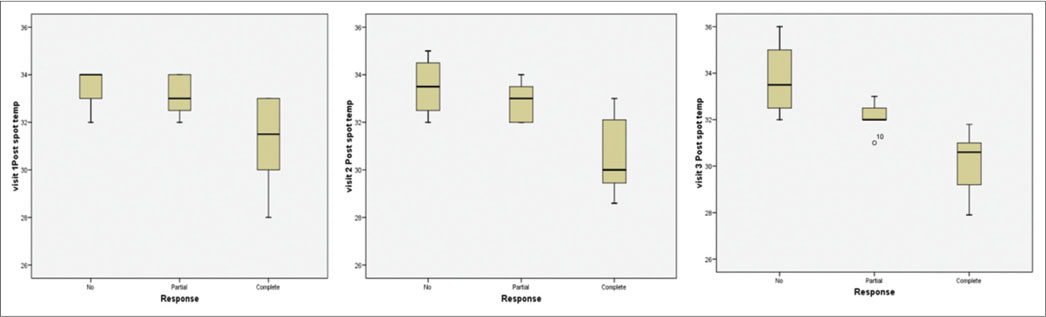
|?Figure. 5? Change of the temperature parameters measured by thermography on the clinical response status was compared|
Among all the methods, there was a trend that the postcool temperature was adequate that this temperature information can be used for monitoring the therapy. We assumed that the combined use of precool and postcool temperature modalities may provide valuable insight to predict treatment response better than separate modality alone. However, results confirm that the postcool temperature can be used for accurate measurement of the treatment response. Hence, assessment of response to chemotherapy in breast carcinoma during neoadjuvant setting can be monitored using TMG.
Although this study was not specifically focused on the application of thermographic imaging to monitor drug toxicities, the relationship between body weight losses would suggest that thermographic imaging could also be utilized to monitor overt treatment toxicity.
Infrared imaging is hazard free, noninvasive method, patient-friendly, and the cost is very low. These features, together with its early detection capability, have enabled infrared imaging to be a strong candidate as a complementary imaging modality to traditional mammography. There was no standard pattern or reporting system developed in TMG as like digital X-ray mammogram. However, for the individual patient, TMG picture was same and was reproducible. Hence, any subtle changes in temperature during the course of chemotherapy were monitored.
The main limitation of this study is the small sample size. Studies are ongoing so that future work can assess the relationship between temperature with different neoadjuvant setting and clinical outcomes in a larger patient population.
Conclusion
This preliminary study suggests that the thermography infrared technique has a potential for monitoring neoadjuvant treatment response in breast cancer patients. In addition, postcool temperature measurement may offer an early indication of the physiological changes happening in the breast tissue in response to neoadjuvant therapy. These findings combined with the improved thermography infrared technique support further for monitoring breast cancer treatment response.
Conflict of Interest
There are no conflicts of interest.
Acknowledgements
The authors wish to thank the patients who participated in the study. We are grateful to Tuscano equipments private limited, a subsidiary of CURA Healthcare for creating this research opportunity by building an innovative product, MAMRIT, now referred as illumina360?. Our sincere thanks to the entire team for their technical support and a special mention to the product team Ms. Aarthee M and Mr. Senthil A for their outstanding contribution in thermography analysis and data collation and Dr. R. Karthick for statistical analysis of the data. This work was supported by grants from BIRAC (Biotechnology Industry Research Assistance Council, A Govt. of India enterprise) through their Biotechnology Industry Partnership Programme (BIPP).
References
- Head JF, Wang F, Lipari CA, Elliott RL.?The important role of infrared imaging in breast cancer. IEEE Eng Med Biol Mag 2000; 19: 52-7
- Foster KR.?Thermographic detection of breast cancer. IEEE Eng Med Biol Mag 1998; 17: 10-4
- Arena F, Barone C, Dicicco T.?Use of infrared imaging in enhanced breast cancer detection and monitoring of the clinical response to treatment. Proceedings of the 25th Annual International Conference of the IEEE EMBS: 17-21 September. 2003. Cancun: Mexico Edited by Engineering in Medicine and Biology Society; 2003: 1129-32
- Threatt B, Norbeck JM, Ullman NS, Kummer R, Roselle PF.?Thermography and breast cancer an analysis of a blind reading. Ann N Y Acad Sci 1980; 335: 501-27
- Ng EY, Sudharsan NM.?Computer simulation in conjunction with medical thermography as an adjunct tool for early detection of breast cancer. BMC Cancer 2004; 4: 17
- Parisky YR, Sardi A, Hamm R, Hughes K, Esserman L, Rust S. et al.?Efficacy of computerized infrared imaging analysis to evaluate mammographically suspicious lesions. AJR Am J Roentgenol 2003; 180: 263-9
- Nomura Y, Takeda M, Hattori T.?Infrared thermometry in diagnosis of breast diseases. Gan No Rinsho 1969; 15: 191-6
- Ng EY.?A review of thermography as promising noninvasive detection modality for breast tumour. Int J Therm Sci 2009; 48: 849-59
- Ng EY, Sudharsan NM.?Numerical modeling in conjunction with thermography as an adjunct tool for breast tumour detection. BMC Cancer 2004; 4: 1-26
- Ammer K, Ring EF.?Standard procedures for infrared imaging in medicine. In: Biomedical Engineering Handbook. Ch.32 CRC Press, Taylor & Francis Group; USA: 2006: 1-14
- Amalu WC, Hobbins WB, Head JF, Elliott RL.?Infrared imaging of the breast ? An overview. In: Biomedical Engineering Handbook. Ch.25 CRC Press, Taylor & Francis Group; USA: 2006: 1-36
- Wiecek B, Wiecek M, Strakowski R, Jakubowska T, Ng EY.?Wavelet-based thermal image classification for breast screening and other medical applications. In: Ng EY, Acharya RU, Suri JS. editors Performance Evaluation Techniques in Multimodality Breast Cancer Screening, Diagnosis and Treatment. American Scientific Publishers; CA, USA: 2010
- Qi H, Kuruganti PT, Snyder WE.?Detecting breast cancer from thermal infrared images by asymmetry analysis. In: Biomedical Engineering Handbook. Ch. 27 CRC Press, Taylor & Francis Group; USA: 2006: 1-14
- Ring EF, Ammer K.?The technique of infra red imaging in medicine. Thermol Int 2000; 10: 7-14
- Kaufmann M, von Minckwitz G, Bear HD, Buzdar A, McGale P, Bonnefoi H. et al.?Recommendations from an international expert panel on the use of neoadjuvant (primary) systemic treatment of operable breast cancer: New perspectives 2006. Ann Oncol 2007; 18: 1927-34
- Rastogi P, Anderson SJ, Bear HD, Geyer CE, Kahlenberg MS, Robidoux A. et al.?Preoperative chemotherapy: Updates of national surgical adjuvant breast and bowel project protocols B-18 and B-27. J Clin Oncol 2008; 26: 778-85
- Fisher ER, Wang J, Bryant J, Fisher B, Mamounas E, Wolmark N. et al.?Pathobiology of preoperative chemotherapy: Findings from the national surgical adjuvant breast and bowel (NSABP) protocol B-18. Cancer 2002; 95: 681-95
- Symmans WF, Peintinger F, Hatzis C, Rajan R, Kuerer H, Valero V. et al.?Measurement of residual breast cancer burden to predict survival after neoadjuvant chemotherapy. J Clin Oncol 2007; 25: 4414-22
- Caudle AS, Gonzalez-Angulo AM, Hunt KK, Liu P, Pusztai L, Symmans WF. et al.?Predictors of tumor progression during neoadjuvant chemotherapy in breast cancer. J Clin Oncol 2010; 28: 1821-8
- Cocconi G, Di Blasio B, Alberti G, Bisagni G, Botti E, Peracchia G. et al.?Problems in evaluating response of primary breast cancer to systemic therapy. Breast Cancer Res Treat 1984; 4: 309-13
- Segel MC, Paulus DD, Hortobagyi GN.?Advanced primary breast cancer: Assessment at mammography of response to induction chemotherapy. Radiology 1988; 169: 49-54
- Moskovic EC, Mansi JL, King DM, Murch CR, Smith IE.?Mammography in the assessment of response to medical treatment of large primary breast cancer. Clin Radiol 1993; 47: 339-44
- Facey K, Bradbury I, Laking G, Payne E.?Overview of the clinical effectiveness of positron emission tomography imaging in selected cancers. Health Technol Assess 2007; 11: iii-iv, xi-267
- Gilbert FJ.?Breast cancer screening in high risk women. Cancer Imaging 2008; 8: S6-9
Address for correspondence
Publication History
Article published online:
24 May 2021
? 2019. Indian Society of Medical and Paediatric Oncology. This is an open access article published by Thieme under the terms of the Creative Commons Attribution-NonDerivative-NonCommercial-License, permitting copying and reproduction so long as the original work is given appropriate credit. Contents may not be used for commercial purposes, or adapted, remixed, transformed or built upon. (https://creativecommons.org/licenses/by-nc-nd/4.0/).
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India

|?Figure. 1? Correlation of sonomammogram with thermo mammogram|

|?Figure. 2? Thermo mammographic images of complete response sample cases

|?Figure. 3? Thermo mammographic images of partial response sample case

|?Figure. 4? Thermo mammographic images of no response sample cases
References
- Head JF, Wang F, Lipari CA, Elliott RL.?The important role of infrared imaging in breast cancer. IEEE Eng Med Biol Mag 2000; 19: 52-7
- Foster KR.?Thermographic detection of breast cancer. IEEE Eng Med Biol Mag 1998; 17: 10-4
- Arena F, Barone C, Dicicco T.?Use of infrared imaging in enhanced breast cancer detection and monitoring of the clinical response to treatment. Proceedings of the 25th Annual International Conference of the IEEE EMBS: 17-21 September. 2003. Cancun: Mexico Edited by Engineering in Medicine and Biology Society; 2003: 1129-32
- Threatt B, Norbeck JM, Ullman NS, Kummer R, Roselle PF.?Thermography and breast cancer an analysis of a blind reading. Ann N Y Acad Sci 1980; 335: 501-27
- Ng EY, Sudharsan NM.?Computer simulation in conjunction with medical thermography as an adjunct tool for early detection of breast cancer. BMC Cancer 2004; 4: 17
- Parisky YR, Sardi A, Hamm R, Hughes K, Esserman L, Rust S. et al.?Efficacy of computerized infrared imaging analysis to evaluate mammographically suspicious lesions. AJR Am J Roentgenol 2003; 180: 263-9
- Nomura Y, Takeda M, Hattori T.?Infrared thermometry in diagnosis of breast diseases. Gan No Rinsho 1969; 15: 191-6
- Ng EY.?A review of thermography as promising noninvasive detection modality for breast tumour. Int J Therm Sci 2009; 48: 849-59
- Ng EY, Sudharsan NM.?Numerical modeling in conjunction with thermography as an adjunct tool for breast tumour detection. BMC Cancer 2004; 4: 1-26
- Ammer K, Ring EF.?Standard procedures for infrared imaging in medicine. In: Biomedical Engineering Handbook. Ch.32 CRC Press, Taylor & Francis Group; USA: 2006: 1-14
- Amalu WC, Hobbins WB, Head JF, Elliott RL.?Infrared imaging of the breast ? An overview. In: Biomedical Engineering Handbook. Ch.25 CRC Press, Taylor & Francis Group; USA: 2006: 1-36
- Wiecek B, Wiecek M, Strakowski R, Jakubowska T, Ng EY.?Wavelet-based thermal image classification for breast screening and other medical applications. In: Ng EY, Acharya RU, Suri JS. editors Performance Evaluation Techniques in Multimodality Breast Cancer Screening, Diagnosis and Treatment. American Scientific Publishers; CA, USA: 2010
- Qi H, Kuruganti PT, Snyder WE.?Detecting breast cancer from thermal infrared images by asymmetry analysis. In: Biomedical Engineering Handbook. Ch. 27 CRC Press, Taylor & Francis Group; USA: 2006: 1-14
- Ring EF, Ammer K.?The technique of infra red imaging in medicine. Thermol Int 2000; 10: 7-14
- Kaufmann M, von Minckwitz G, Bear HD, Buzdar A, McGale P, Bonnefoi H. et al.?Recommendations from an international expert panel on the use of neoadjuvant (primary) systemic treatment of operable breast cancer: New perspectives 2006. Ann Oncol 2007; 18: 1927-34
- Rastogi P, Anderson SJ, Bear HD, Geyer CE, Kahlenberg MS, Robidoux A. et al.?Preoperative chemotherapy: Updates of national surgical adjuvant breast and bowel project protocols B-18 and B-27. J Clin Oncol 2008; 26: 778-85
- Fisher ER, Wang J, Bryant J, Fisher B, Mamounas E, Wolmark N. et al.?Pathobiology of preoperative chemotherapy: Findings from the national surgical adjuvant breast and bowel (NSABP) protocol B-18. Cancer 2002; 95: 681-95
- Symmans WF, Peintinger F, Hatzis C, Rajan R, Kuerer H, Valero V. et al.?Measurement of residual breast cancer burden to predict survival after neoadjuvant chemotherapy. J Clin Oncol 2007; 25: 4414-22
- Caudle AS, Gonzalez-Angulo AM, Hunt KK, Liu P, Pusztai L, Symmans WF. et al.?Predictors of tumor progression during neoadjuvant chemotherapy in breast cancer. J Clin Oncol 2010; 28: 1821-8
- Cocconi G, Di Blasio B, Alberti G, Bisagni G, Botti E, Peracchia G. et al.?Problems in evaluating response of primary breast cancer to systemic therapy. Breast Cancer Res Treat 1984; 4: 309-13
- Segel MC, Paulus DD, Hortobagyi GN.?Advanced primary breast cancer: Assessment at mammography of response to induction chemotherapy. Radiology 1988; 169: 49-54
- Moskovic EC, Mansi JL, King DM, Murch CR, Smith IE.?Mammography in the assessment of response to medical treatment of large primary breast cancer. Clin Radiol 1993; 47: 339-44
- Facey K, Bradbury I, Laking G, Payne E.?Overview of the clinical effectiveness of positron emission tomography imaging in selected cancers. Health Technol Assess 2007; 11: iii-iv, xi-267
- Gilbert FJ.?Breast cancer screening in high risk women. Cancer Imaging 2008; 8: S6-9


 PDF
PDF  Views
Views  Share
Share

